Incidence of HCC after HCV treatment with DAAs: ERCHIVES
Li DK. Hepatology 2018;67:2244-53
Retrospective cohort study
- HCV infected Veterans, 2002-2016
- Exclusion: HIV or HBV co-infection, HCC prior to baseline, missing information on outcome/follow-up
- Data collection: demographics, clinical, laboratory, prescriptions
- Baseline
- Treatment group (IFN-PEG + RBV or DAA = 28 days ): date of HCV treatment initiation
- Untreated group: duration of HCV infection before treatment initiation in the corresponding treated person
- SVR: undetectable HCV RNA at least 12 weeks after end of initial treatment
Primary outcome
- Incident HCC = 3 months after baseline
- Primary analysis performed in persons with baseline cirrhosis (FIB-4 > 3.5)
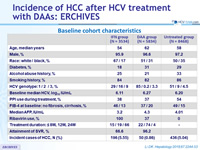
Baseline cohort characteristics
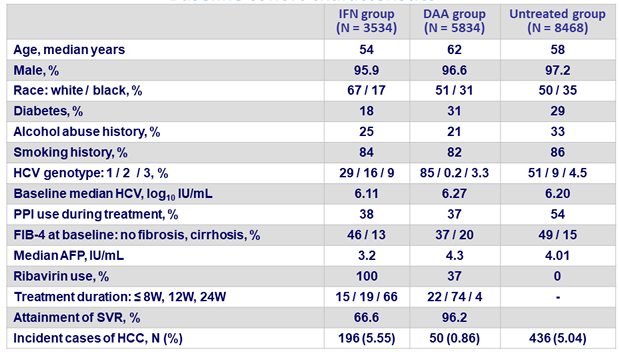
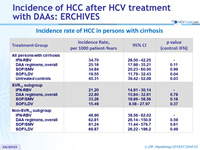
Incidence rate of HCC in persons with cirrhosis
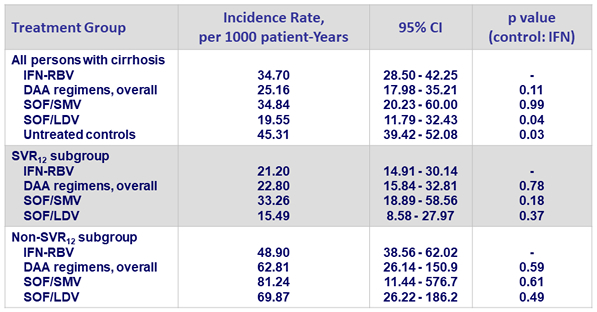
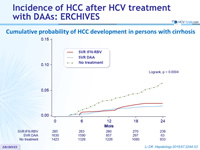
Cumulative probability of HCC development in persons with cirrhosis
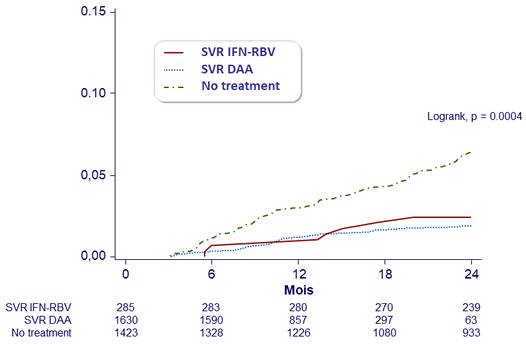
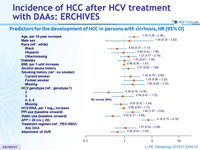
Predictors for the development of HCC in persons with cirrhosis, HR (95% CI)
Conclusion
- DAA treatment is not associated with a higher risk of HCC in persons with cirrhosis with chronic HCV infection in the short term
- Previously reported higher rates of HCC associated with DAA treatment may be explained by both the presence of relatively fewer baseline HCC risk factors in persons treated with IFN-RBV as well as selection bias, given that DAA regimens were used to treat persons at higher risk for developing HCC