Clinical outcome after SVR: ANRS CO22 HEPATHER
Carrat F, AASLD 2017, Abs. LB-28
Design
- Observational prospective cohort (France)
- 12 502 HCV-infected patients
- Exclusion criteria: HBV co-infection, history of decompensated cirrhosis, HCC or liver transplant, treatment with IFN-RBV ± 1st generation PI, no follow-up
- Survival time since enrolment or start of DAA (censoring date: July 1st, 2017, or death, or HCC, or decompensated cirrhosis)
- Cox proportional hazards models with Inverse Probability of Treatment Weighting (IPTW) to quantify the impact of DAA (as a time dependent-covariate) on clinical outcomes
- IPTW scores were obtained from a logistic model linking treatment with baseline covariates that confounded the treatment-outcome relationship
Study population
- 6 460 patients received DAA
- 8 462 patient-years of follow up, occurrence of 90 deaths, 164 HCC and 77 decompensation
- 2 835 patients did not receive DAA
- 10 040 patient-years of follow up, occurrence of 78 deaths, 57 HCC and 35 decompensation
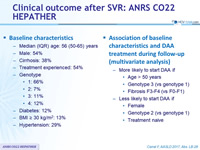
Baseline characteristics
- Median (IQR) age: 56 (50-65) years
- Male: 54%
- Cirrhosis: 38%
- Treatment experienced: 54%
- Genotype
- 1: 66%
- 2: 7%
- 3: 11%
- 4: 12%
- Diabetes: 12%
- BMI = 30 kg/m 2 : 13%
- Hypertension: 29%
Association of baseline characteristics and DAA treatment during follow-up (multivariate analysis)
- More likely to start DAA if
- Age > 50 years
- Genotype 3 (vs genotype 1)
- Fibrosis F3-F4 (vs F0-F1)
- Less likely to start DAA if
- Female
- Genotype 2 (vs genotype 1)
- Treatment naive
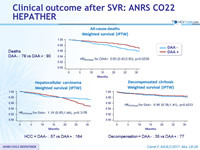
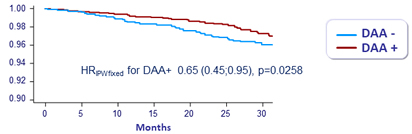
All-cause-deaths Weighted survival (IPTW)
Hepatocellular carcinoma Weighted survival (IPTW)
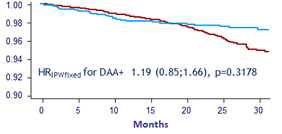
HCC = DAA - : 57 vs DAA + : 164
Decompensated cirrhosis Weighted survival (IPTW)
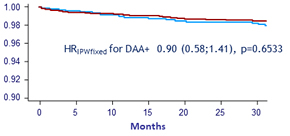
Decompensation = DAA - : 35 vs DAA + : 77
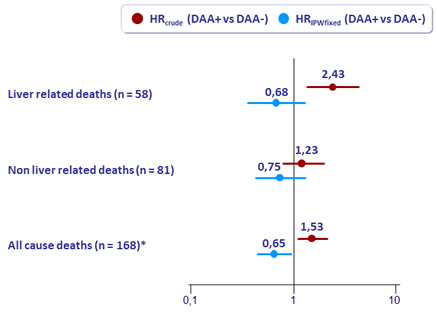
Conclusions
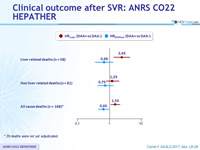
- DAA was associated with
- a decreased risk of death
- The decrease in deaths was more pronounced for liver-related deaths than for non liver-related deaths
- and no increased risk of HCC and decompensation
- a decreased risk of death