Reduction in HCC risk after SVR:
Veterans Affairs
Ioannou GN, AASLD 2017, Abs. 142
Design
- Retrospective cohort (Veterans Affairs, 167 medical centers USA)
- Follow-up start : 180 days after initiation of the 1st antiviral treatment, 1999-2015 ; end of follow-up : June 15, 2017 or death or loss to follow-up
- IFN ± RBV, n = 35 871
- DAA + IFN-based, n = 4 535
- DAA only, n = 21 949
- Exclusion of patients with HCC prior to antiviral treatment or occurring < 180 days post-initiation of antiviral treatment
- After a mean follow-up of 6.1 years, incident HCC = 3271
- Cox proportional hazards regression adjusted for potential confounders
- SVR12 vs treatment failure
- Treatment with DAA vs IFN
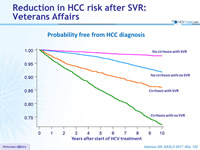
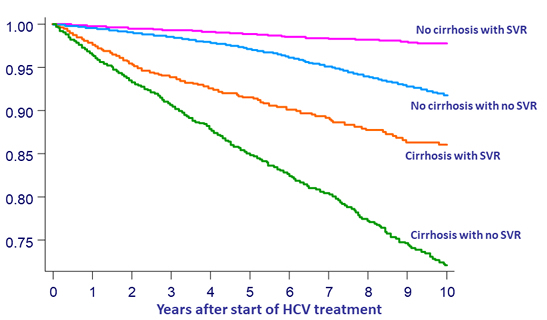
Probability free from HCC diagnosis
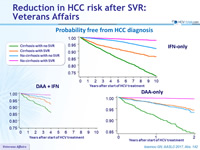
Probability free from HCC diagnosis
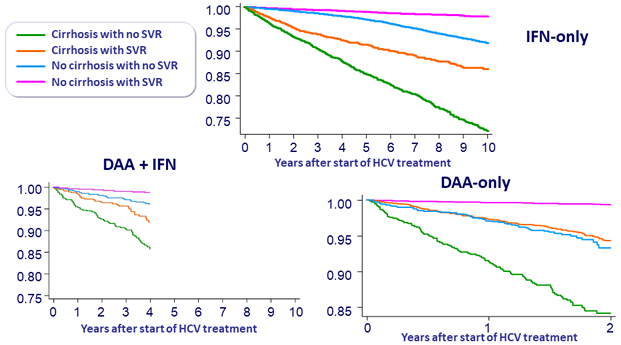
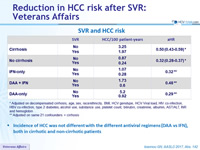
SVR and HCC risk
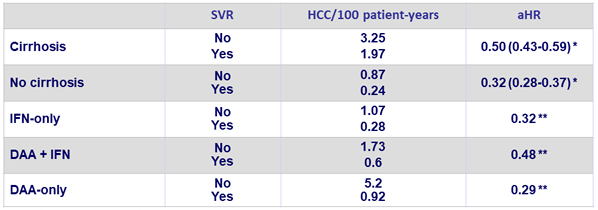
* Adjusted on decompensated cirrhosis, age, sex, race/ethnicity, BMI, HCV genotype, HCV Viral load, HIV co-infection, HBV co-infection, type 2 diabetes, alcohol use, substance use, platelet count, bilirubin, creatinine, albumin, AST/ALT, INR and hemoglobin
** Adjusted on same 21 confounders + cirrhosis
- Incidence of HCC was not different with the different antiviral regimens (DAA vs IFN), both in cirrhotic and non-cirrhotic patients
Conclusions
- SVR is associated with a reduction in HCC risk
- Reduction of risk is independent of regimen
- 68% with IFN-only regimens
- 52% with DAA (boceprevir, telaprevir or sofosbuvir) + IFN
- 71% with DAA-only regimens
- Reduction of risk is similar in cirrhotic and non-cirrhotic patients
- Reduction of risk is independent of regimen
- DAA is not associated with increased HCC risk compared with IFN