Tropifexor (LJN452) in NASH
FLIGHT-FXR Study: Tropifexor (LJN452)
in NASH: phase 2b (interim results)
Sanyal AJ, AASLD 2018, Abs. LB-23
Design
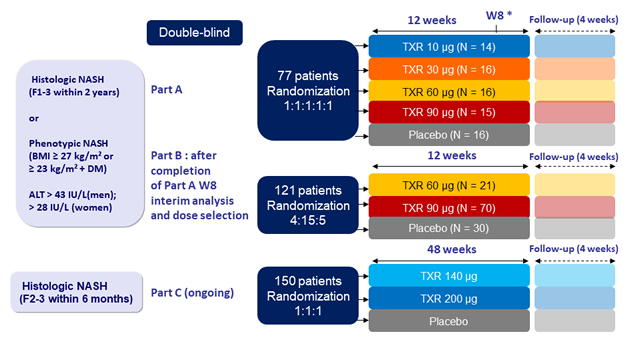
Endpoints
- Safety, liver fat content (MRI-PDFF), liver biochemistry
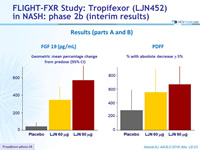
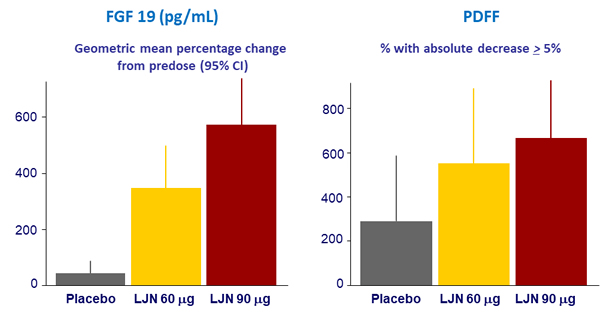
Results (parts A and B)
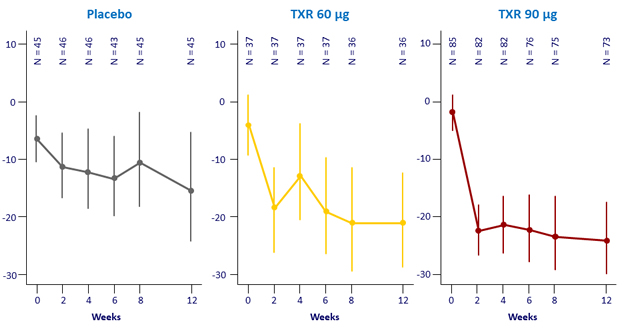
Geometric mean % change from baseline in ALT (IU/L), 95% CI – Parts A and B
Geometric mean % change from baseline in GGT (IU/L), 95% CI – Parts A and B
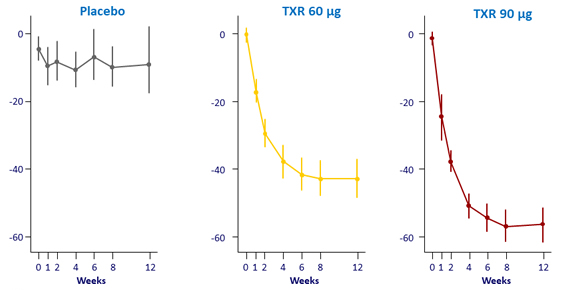
Safety
- Comparable rates of adverse events, including pruritus, for Tropifexor and placebo
- Mild dose response increase of LDL and decrease of HDL, unchanged triglycerides