VK2809 in NAFLD
VK2809 in NAFLD: a phase 2 study
Loomba R, AASLD 2018, Abs. LB4
Design
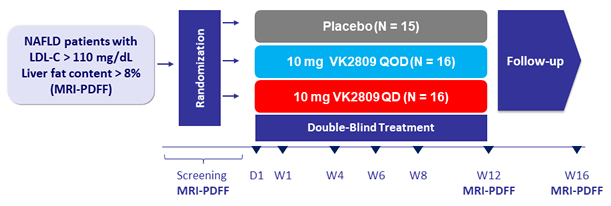
- VK2809: selected liver-targeted thyroid receptor β agonist
Endpoints
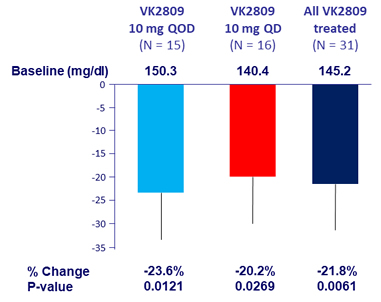
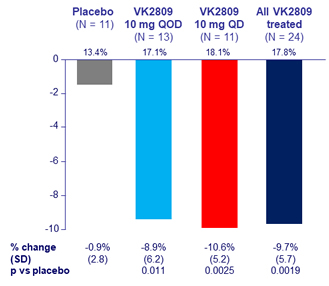
- Primary: change in LDL-cholesterol vs placebo
- Secondary: change in liver fat by MRI-PDFF
- Exploratory: changes in atherogenic lipoproteins
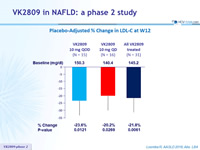
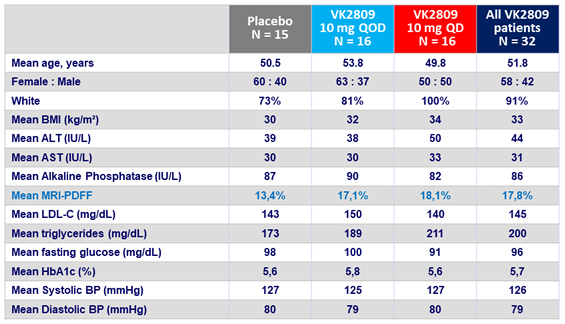
Baseline characteristics
Placebo-Adjusted % Change in LDL-C at W12
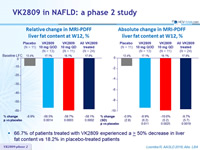
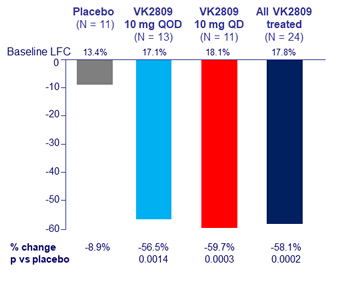
Relative change in MRI-PDFF liver fat content at W12, %
Absolute change in MRI-PDFF liver fat content at W12, %
- 66.7% of patients treated with VK2809 experienced a ≥ 50% decrease in liver fat content vs 18.2% in placebo-treated patients
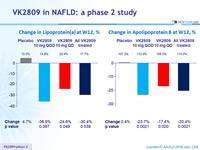
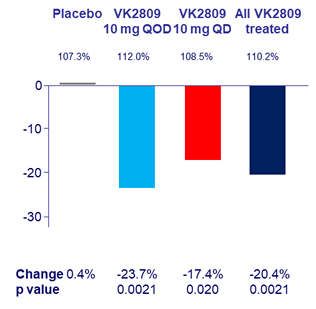
Change in Lipoprotein(a) at W12, %
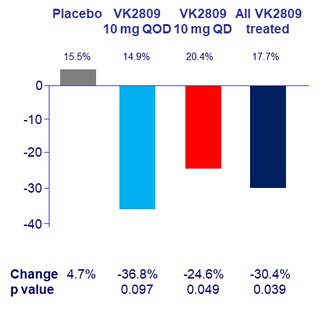
Change in Apolipoprotein B at W12, %
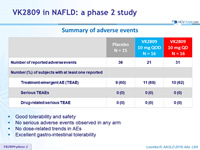
Summary of adverse events
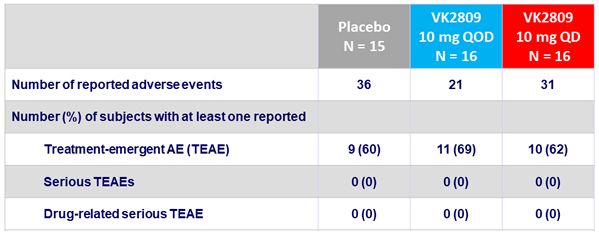
- Good tolerability and safety
- No serious adverse events observed in any arm
- No dose-related trends in AEs
- Excellent gastro-intestinal tolerability
Summary
- VK2809 produced robust reduction in liver fat on MRI-PDFF in NAFLD patients after 12 weeks of oral dosing
- Up to 91% of patients dosed with VK2809 experienced a response as demonstrated by liver fat reductions ≥ 30% relative to baseline ; 67% experienced liver fat reductions ≥ 50%
- VK2809 produced significant reduction in LDL-C, triglycerides, Apo B, and Lp(a) relative to placebo in NAFLD patients
- VK2809 was safe and well-tolerated