ARREST Study: Phase 2b of Aramchol in NASH
Ratziu V, AASLD 2018, Abs. LB5
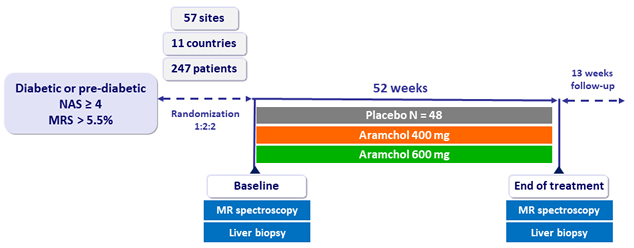
Design
- Aramchol: stearoyl-CoA desaturase receptor modulator
Endpoints
- Primary: absolute change in liver triglycerides
- Secondary: decrease in fibrosis ≥ 1, NASH resolution
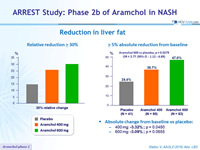
Reduction in liver fat
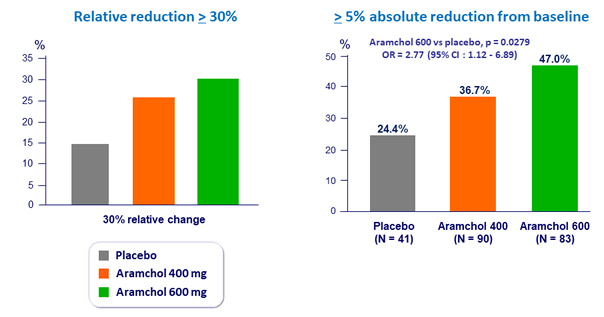
- Absolute change from baseline vs placebo:
- 400 mg: -3.32% ; p = 0.0450
- 600 mg: -3.09% ; p = 0.0655
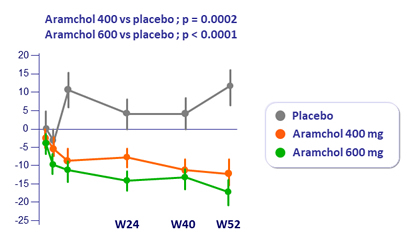
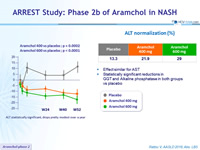
ALT statistically significant, drops pretty modest over a year
ALT normalization (%)

- Effect similar for AST
- Statistically significant reductions in GGT and Alkaline phosphatase in both groups vs placebo
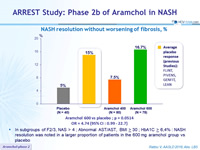
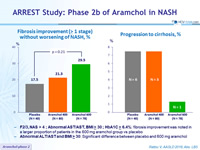
NASH resolution without worsening of fibrosis, %
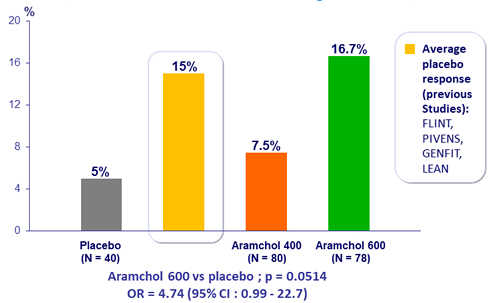
- In subgroups of F2/3, NAS > 4 ; Abnormal AST/AST, BMI > 30 ; HbA1C > 6,4% : NASH resolution was noted in a larger proportion of patients in the 600 mg aramchol group vs placebo
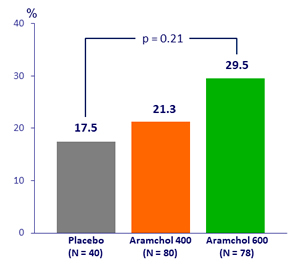
Fibrosis improvement (> 1 stage) without worsening of NASH, %
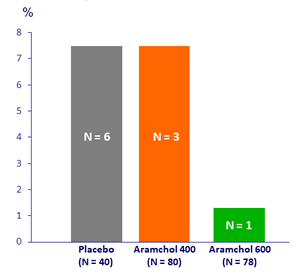
Progression to cirrhosis, %
- F2/3, NAS > 4 ; Abnormal AST/AST, BMI ≥ 30 ; HbA1C ≥ 6.4%: fibrosis improvement was noted in a larger proportion of patients in the 600 mg aramchol group vs placebo
- Abnormal ALT/AST and BMI ≥ 30: Significant difference between placebo and 600 mg aramchol