CENTAUR Study: cenicriviroc in NASH (phase 2b)
Friedman SL, Hepatology 2018;67:1754-67, Ratziu , EASL 2018, Abs. GS-002
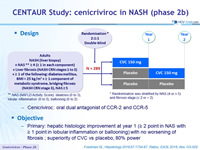
Design
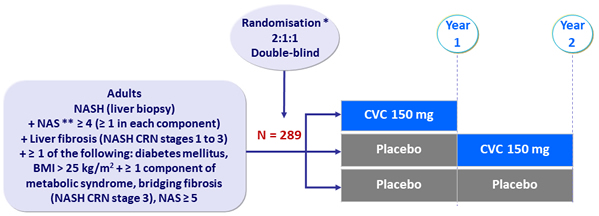
* Randomisation was stratified by NAS (4 or = 5) and fibrosis stage (= 2 or > 2)
** NAS ( NAFLD Activity Score): steatosis (0 to 3), lobular inflammation (0 to 3), ballooning (0 to 2)
- Cenicriviroc : oral dual antagonist of CCR-2 and CCR-5
Objective
- Primary: hepatic histologic improvement at year 1 (= 2 point in NAS with = 1 point in lobular inflammation or ballooning) with no worsening of fibrosis ; superiority of CVC vs placebo, 80% power
Baseline characteristics and disposition
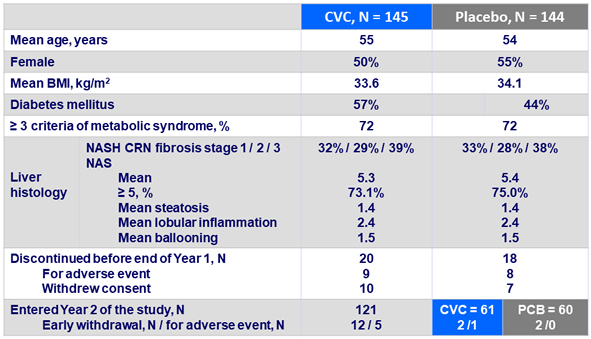
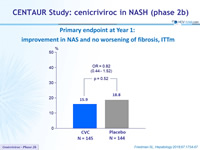
Primary endpoint at Year 1: improvement in NAS and no worsening of fibrosis, ITTm
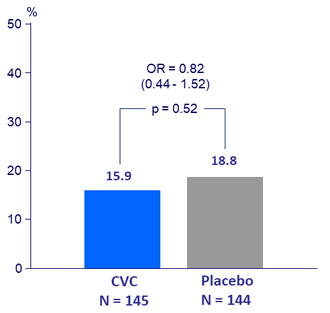
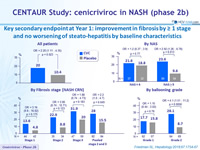
Key secondary endpoint at Year 1: improvement in fibrosis by = 1 stage and no worsening of steato -hepatitis by baseline characteristics
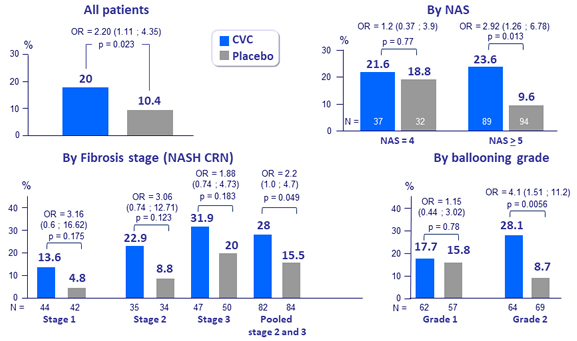
Improvement in fibrosis by = 1 stage and no worsening of steato-hepatitis
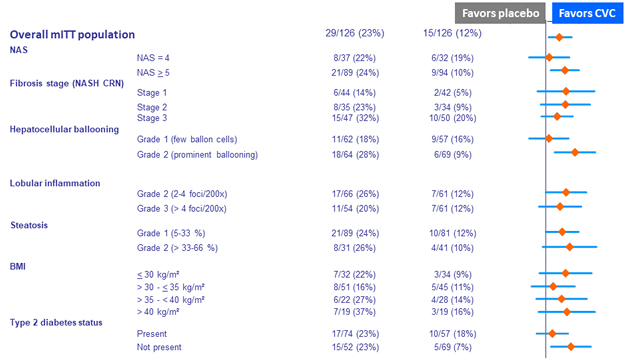
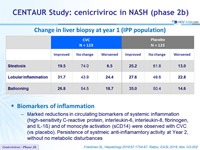
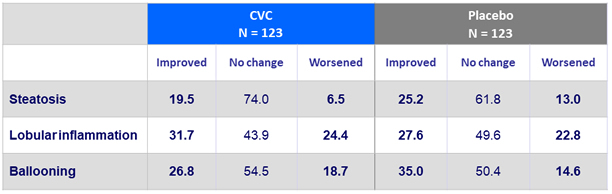
Change in liver biopsy at year 1 (IPP population)
Biomarkers of inflammation
- Marked reductions in circulating biomarkers of systemic inflammation (high-sensitivity C-reactive protein, interleukin-6, interleukin-8, fibrinogen, and IL-1ß) and of monocyte activation (sCD14) were observed with CVC (vs placebo). Persistence of systmeic anti- inflamamtory activity at Year 2, without no metabolic disturbances
Main outcomes at Year 2
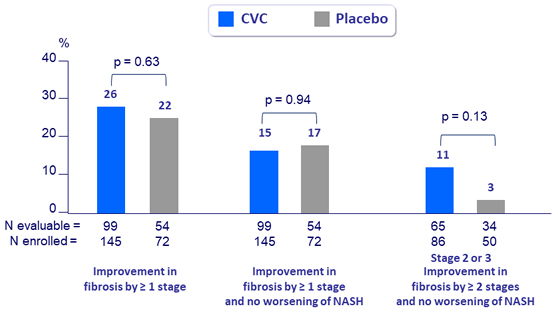
- Improvement in fibrosis (= 1 stage)
- Improvement in fibrosis (= 1 or = 2 stages) and no worsening of NASH
- Maintenance of antifibrotic response from Year 1 to Year 2 (groups with 2 years CVC vs 2 years of placebo)
- Effects of 2 years of CVC treatment (groups with 2 years CVC vs 2 years of placebo)
- Effects of 1 year of CVC treatment (immediate or deferred CVC vs placebo)
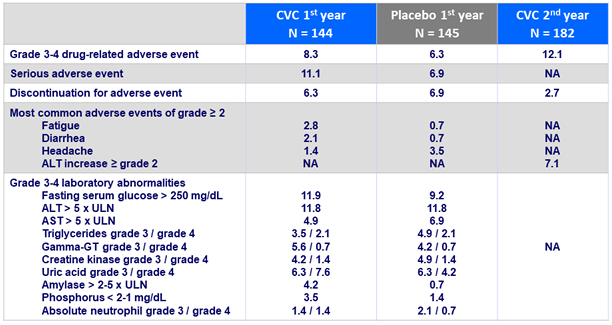
- Safety and tolerability of CVC vs placebo during Year 2
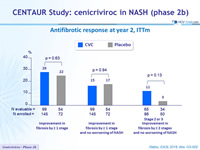
Antifibrotic response at year 2, ITTm
Adverse events and laboratory abnormalities, %
Summary
- CVC showed a significant antifibrotic benefit at year 1 and was well tolerated
- Although the primary endpoint of the study was not met, the fact that the CENTAUR year 1 study results showed that CVC provided clinically meaningful benefits and resulted in twice as many subjects achieving improvement in fibrosis by 1 stage and no worsening of steato -hepatitis as compared to placebo suggests that the study did, in fact, show proof of concept, warranting phase 3 development of CVC
- Year 2 analyses corroborate CVC antifibrotic activity in adults with NADH and liver fibrosis
- Effect more pronounced in stage 3 fibrosis