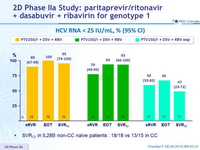
2D Phase IIa Study: paritaprevir /ritonavir + dasabuvir + ribavirin for genotype 1
Exploratory Study of Oral Combination Antiviral Therapy for Hepatitis C
Poordad F. NEJM 2014;368:45-53
Anti-HCV
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Dasabuvir
Ribavirin
Genotype
1
1a
1
1a
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
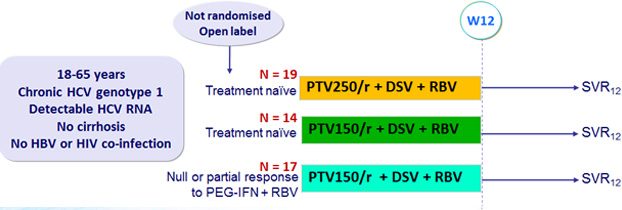
Treatment regimens
- P aritaprevir / rironavir (PTV/r) : PTV 250 or 150 mg qd / ritonavir 100 mg qd (2 tablets)
- Dasabuvir (DSV) : 400 mg bid
- RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or = 75 kg)
Endpoints
- Primary : eRVR (undetectable HCV RNA from W4-W12), with 95% CI
- Secondary : SVR12 (HCV RNA < 25 IU/ mL ), with 95% CI
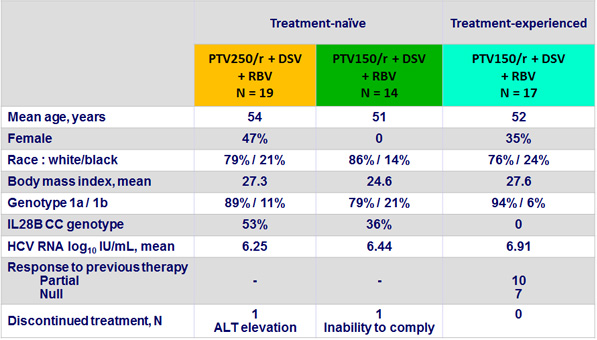
Baseline characteristics and patient disposition
HCV RNA < 25 IU/mL, % (95% CI)
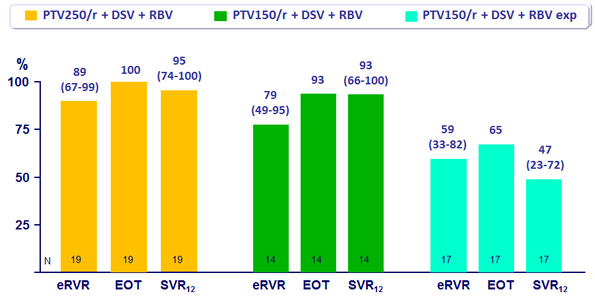
- SVR12 in IL28B non-CC naïve patients : 18/18 vs 13/15 in CC
Virologic breakthrough
- None in naïve patients
- Six (35%) in previous non-responders
Relapse
- None in naïve patients
- 3 in pre-treated patients
Resistance testing (population sequencing ) of the 9 failures
- 8/9 had = 1 mutant resistant variants in NS3 and NS5B
- NS3 : position 168 (N = 8) + 155 (N = 1)
- NS5B : position 316 (N = 2), 414 (N = 3), 554 (N = 2), 556 (N = 4), 559 (N = 1)
- 1 patient had a baseline NS3 168 mutation
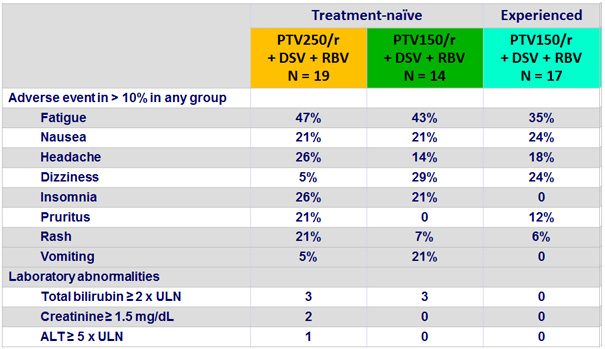
Adverse events, n (%)
Summary
- This preliminary study suggests that the all-oral combination of paritaprevir /r, dasabuvir, and ribavirin for 12 weeks is associated with a sustained virologic response in a high proportion of previously untreated patients with HCV genotype 1 infection
- This regimen is less effective in patients who have HCV genotype 1 infection with a null or partial response to previous therapy
- In most cases, virologic failure was associated with the emergence of variants with substitutions in both NS3 and NS5B, at positions known to confer resistance in vitro to PTV and DSV, respectively
- Discontinuation for adverse event occurred in 1 patient (asymptomatic ALT elevation)