SWIFT-C study: SOF + RBV or LDV/SOF for acute hepatitis C in HIV-infected patients
Naggie S. Clin Infect Dis 2017; 64:1035-42 ; Naggie S. AASLD 2017, Abs. 196
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1
1
Special population
Acute HCV infection
Acute HCV infection
Design
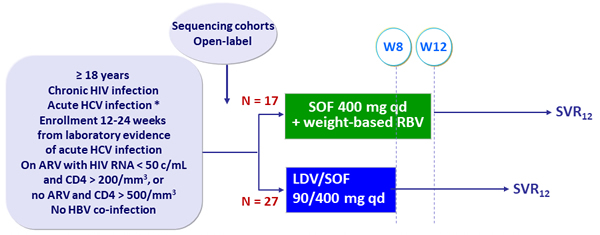
* New detectable HCV RNA + ALT ≥ 5 x ULN if normal in prior 12 months, or ALT ≥ 10 x ULN with no baseline ALT, or negative HCV Ab or RNA in prior 6 months
- RBV dosed twice daily : 1200 mg if ≥ 75 kg, 1000 mg if < 75 kg
Objective
- SVR12 (HCV RNA < 15 UI/ml) with 2-sided 90% CI, 90% power to show that true SVR12> 60% for SOF + RBV 12 weeks
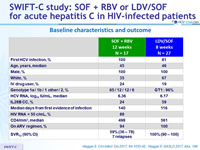
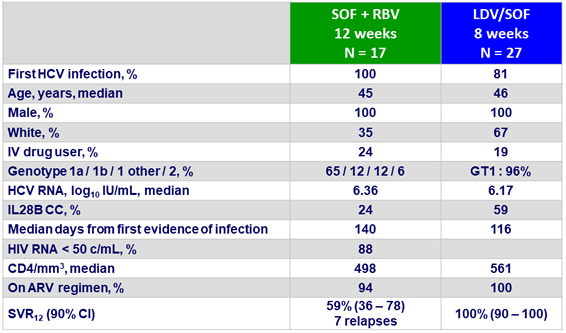
Baseline characteristics and outcome
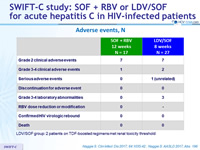
Adverse events, N
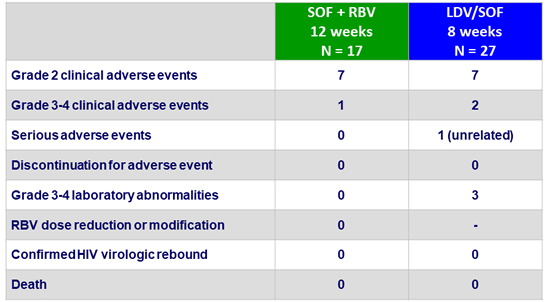
LDV/SOF group: 2 patients on TDF- boosted regimens met renal toxicity threshold
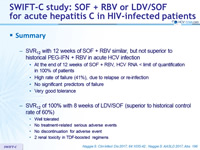
Summary
- SVR12 with 12 weeks of SOF + RBV similar, but not superior to historical PEG-IFN + RBV in acute HCV infection
- At the end of 12 weeks of SOF + RBV, HCV RNA < limit of quantification in 100% of patients
- High rate of failure (41%), due to relapse or re-infection
- No significant predictors of failure
- Very good tolerance
- SVR12 of 100% with 8 weeks of LDV/SOF (superior to historical control rate of 60%)
- Well tolerated
- No treatment-related serious adverse events
- No discontinuation for adverse event
- 2 renal toxicity in TDF-boosted regimens