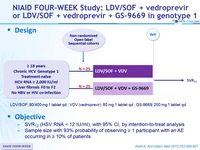
NIAID FOUR-WEEK Study: LDV/SOF + vedroprevir
or LDV/SOF + vedroprevir + GS-9669 in genotype
Kohli A. Ann Intern Med 2015;163:899-907
Anti-HCV
Ledipasvir
Sofosbuvir
Vedroprevir (GS-9451)
Radalbuvir (GS-9669)
Ledipasvir
Sofosbuvir
Vedroprevir (GS-9451)
Radalbuvir (GS-9669)
Genotype
1
1
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
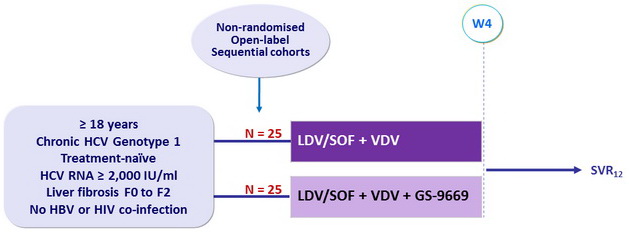
LDV/SOF: 90/400 mg 1 tablet qd ; VDV ( vedroprevir ): 80 mg 1 tablet qd ; GS-9669: 250 mg 1 tablet qd
Objective
- SVR12 (HSV RNA < 12 IU /ml) , with 95% CI, by intention-to-treat analysis
- Sample size with 93% probability of observing = 1 participant with an AE occurring in = 10% of patients
Baseline characteristics and outcome
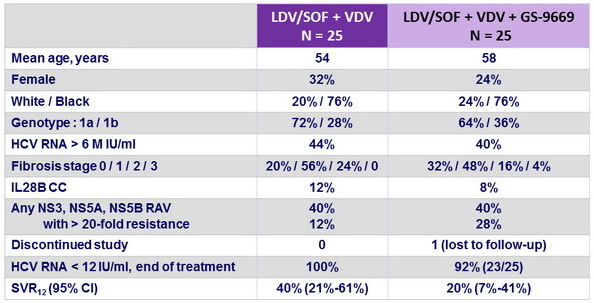
Treatment outcomes by subgroups
- SVR12 higher if lower age, genotype 1b, HCV RNA < 6 M IU/ml
Analysis of Resistance associated variants
- Genotype 1a, N = 33
- NS3 Q80K at baseline (3-fold reduced susceptibility to VDV), N = 10
- Viral relapse: 60% if Q80K+ vs 97% if Q80K-
- NS3 R155K and D168E and/or NS5A L31M, Y93H and Y93N
(> 20-fold reduced susceptibility to VDV and LDV, respectively), N = 10
- Viral relapse: 100% if these RAVS present vs 63% if absent (p = 0.022)
- NS5B M423I (4.6 fold resistance to GS-9669), N = 1
- Viral relapse in this patient
- NS3 Q80K at baseline (3-fold reduced susceptibility to VDV), N = 10
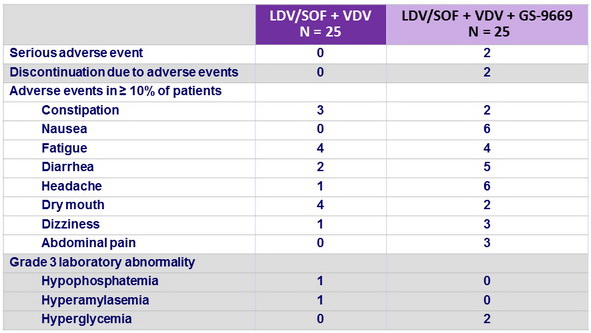
Adverse events, N
Summary
- Treatment for 4 weeks with the all oral combination DAA regimens used in this study seems to have high tolerability but limited response in achieving SVR12 in non cirrhotic treatment-naive patients with HCV genotype 1 infection