ASTRAL-5 study: SOF/VEL in HIV coinfection
Wyles D, Clin Infect Dis 2017 ; 65 :6-12
Anti-HCV
Sofosbuvir
Velpatasvir (GS-5816)
Sofosbuvir
Velpatasvir (GS-5816)
Genotype
1
2
3
4
1
2
3
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Special population
HIV co-infection
HIV co-infection
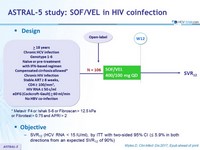
Design
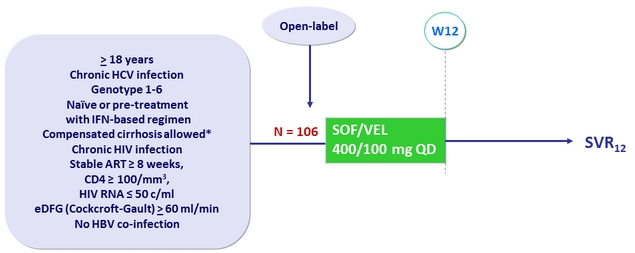
* Metavir F4 or Ishak 5-6 or Fibroscan > 12.5 kPa
or Fibrotest > 0.75 and APRI > 2
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT with two-sided 95% CI (= 5.9% in both directions from an expected SVR12 of 90%)
Baseline characteristics
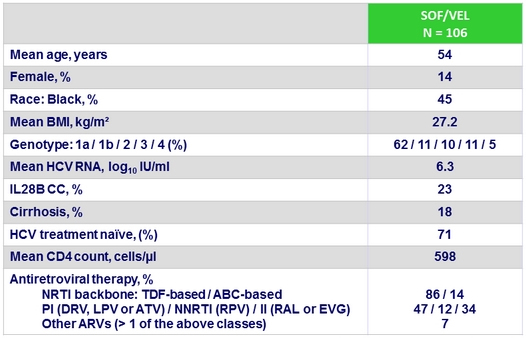
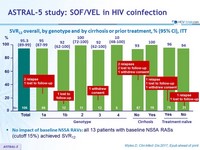
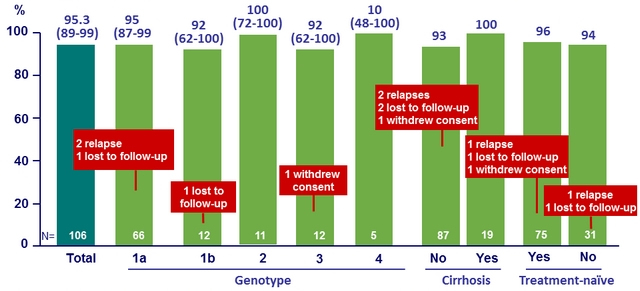
SRV12 overall, by genotype and by cirrhosis or prior treatment, % (95% CI), ITT
- No impact of baseline NS5A RAVs: all 13 patients with baseline NS5A RASs (cutoff 15%) achieved SVR12
Adverse events, %
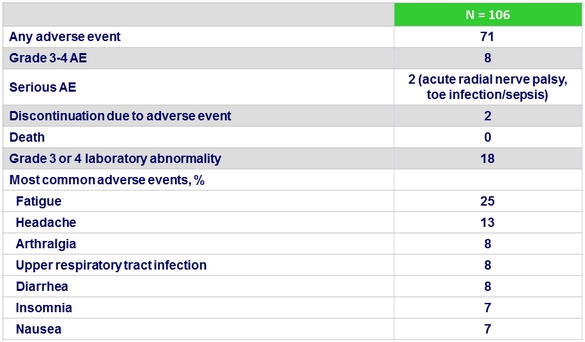
No HIV rebound
Laboratory abnormalities, %
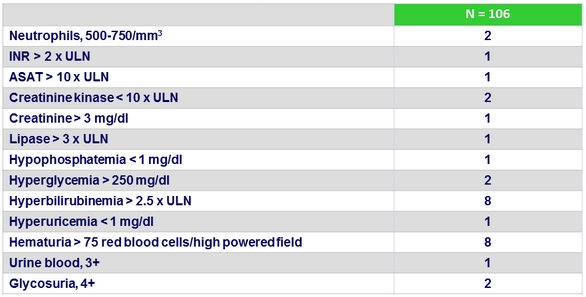
3 patients, all receiving TDF-containing regimens, had a change from baseline of = 0.4 mg/dl in serum creatinine while on treatment
CD4+ counts remained stable and no patient experienced HIV virologic rebound
Summary
- SOF/VEL for 12 weeks resulted in overall 95% SVR12 in HIV coinfected patients
- 100% SVR12 in patients with cirrhosis
- 96% SVR12 in patients with prior failure to HCV therapy
- Presence of baseline RAVs did not impact efficacy
- Treatment was safe and well tolerated, including with TDF-based boosted regimens
- In conclusion, SOF/VEL for 12 weeks provides a simple, safe, and highly effective treatment for patients coinfected with HCV and HIV-1