C-EDGE IBLD: elbasvir/grazoprevir for HCV infected patients with inherited blood disorders (IBLD)
Hezode C, Hepatology 2017 ; 66 :736-45
Anti-HCV
Grazoprevir
Elbasvir
Grazoprevir
Elbasvir
Genotype
1
4
1
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Special population
Inherited blood disorder
Inherited blood disorder
Design
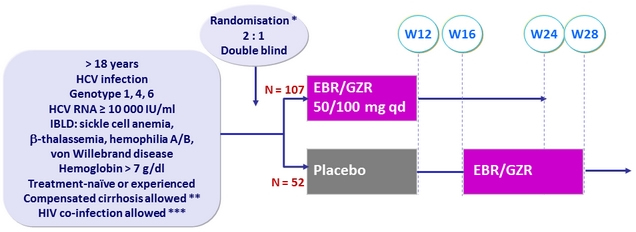
* Fibroscan > 12.5 kPa , or FibroTest > 0.75 + APRI > 2
** On stable ART with TDF or ABC, 3TC/FTC + RAL or DTG or RPV for ≥ 8 weeks,
and CD4 > 200/mm3, and undetectable HIV RNA
Objective
- SVR12 (HCV RNA < 15 IU /ml), by ITT analysis, 99% power to demonstrate superiority to a reference rate of 40% at an overall 1-sided 2.5% alpha level
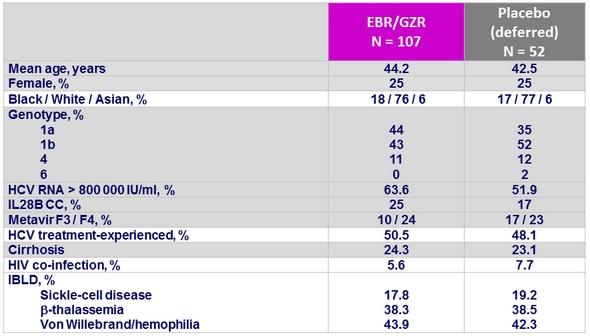
Baseline characteristics
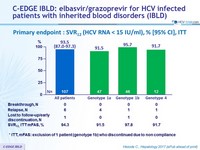
Primary endpoint : SRV12 (HCV RNA < 15 IU /ml), % [95% CI], ITT
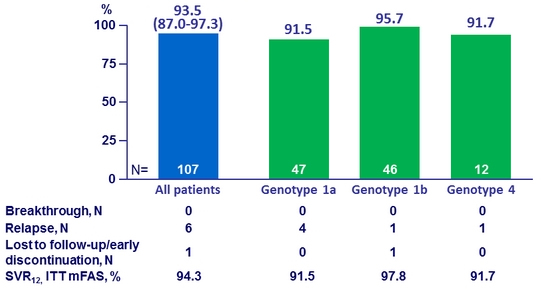
* ITT, mFAS : exclusion of 1 patient (genotype 1b) who discontinued due to non compliance
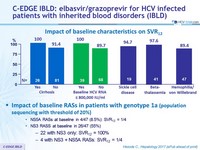
Impact of baseline RASs on
Impact of baseline RASs in patients with genotype 1a (population sequencing with threshold of 20%)
- NS5A RAVs at baseline in 4/47 (8.5%): SVR 12 = 1/4
- NS3 RAVS at baseline in 26/47 (55%)
- 22 with NS3 only: SVR12 = 100%
- 4 with NS3 + NS5A RAVs: SVR12 = 1/4
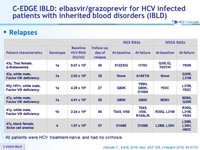
Relapses
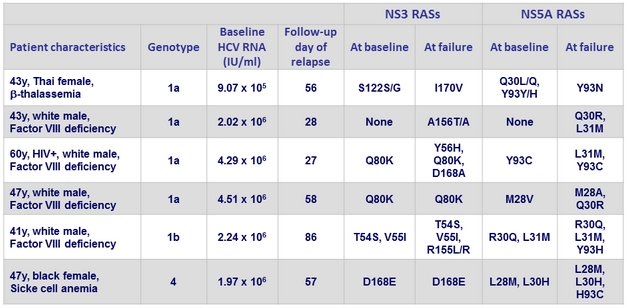
- All patients were HCV treatment-naïve and had no cirrhosis
Adverse events and laboratory abnormalities, %
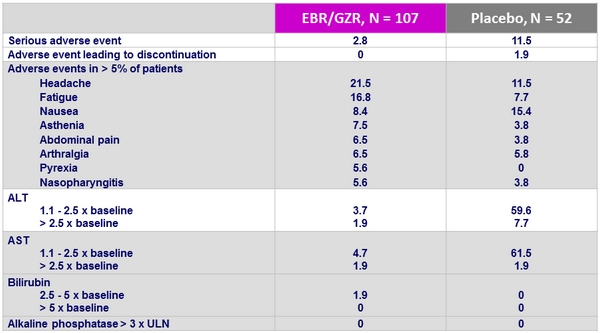
Summary
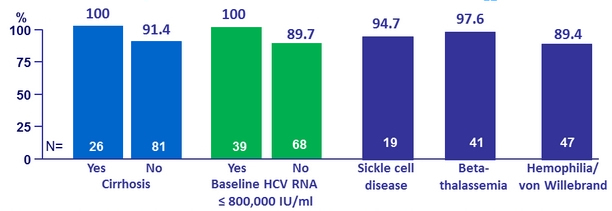
- 12 weeks of EBR/GZR was highly efficacious in patients with inherited blood disorders and HCV genotype1 and 4 infection, and evidenced in various patient subgroups
- Cirrhosis
- HIV co-infection
- All IBLDs
- Lower response in patients with genotype 1a infection with baseline NS5A RASs
- Tolerance was generally good
- No impact on measures of hematology and clotting or on the treatment of the underlying blood disorder