MAGELLAN-I Study, Part 1 : Glecaprevir+ Pibrentasvir in genotype 1 with failure to DAA regimen - Phase II
Poordad F. Hepatology. 2017 ; 66 : 389-97
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
1a
1
1a
Treatment history
PI (NS3)-experienced
NS5A experienced
SOF-experienced
PI (NS3)-experienced
NS5A experienced
SOF-experienced
Cirrhosis
No
No
Design
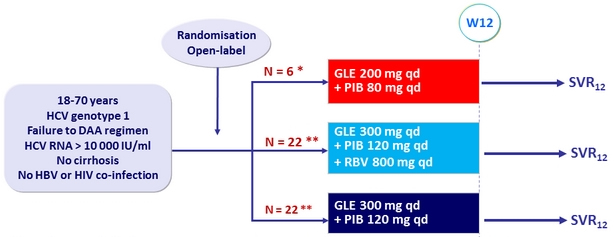
* Low dose arm halted
(based on dose-findings results across all genotypes)
** Randomisation stratified by genotype (1b or non-1b) and prior treatment (NS5A inhibitor-experienced, NS3/4A PI-experienced but NS5A inhibitor-naive, or other)
Objective
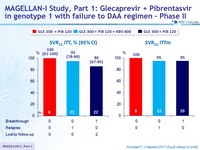
- SVR12 (HCV RNA< 25 IU /ml), by ITT
Baseline characteristics
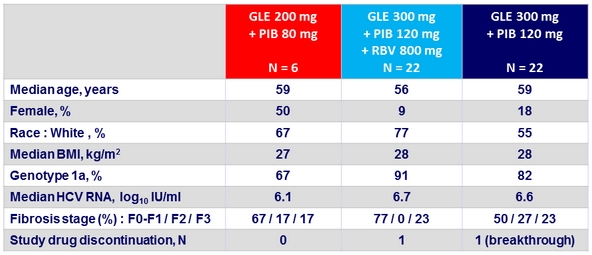
Prior regimen
- PI-experienced , NS5A naïve, n = 25; NS5A-experienced, PI-naïve, n = 8
- PI and NS5A-experienced, n = 17
- SOF-experienced, n = 16
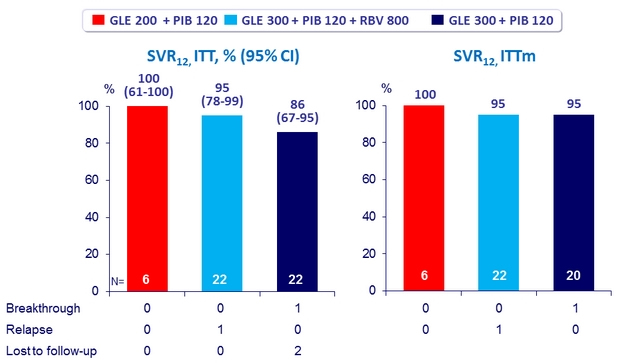
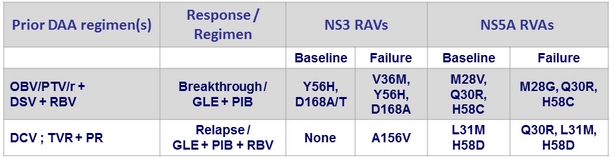
2 virologic failures in genotype 1a
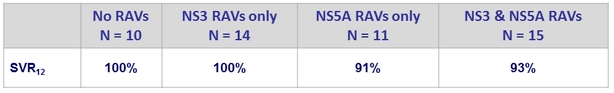
SRV12 (ITTm) according to baseline RAVs
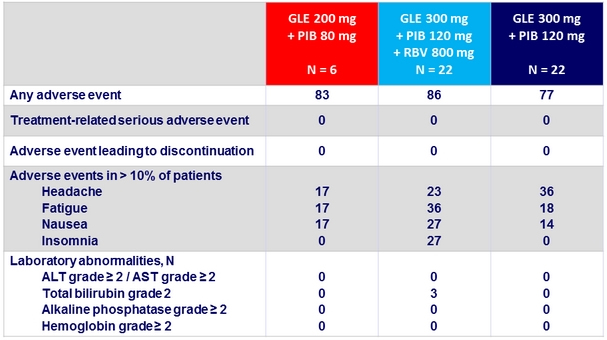
Adverse events and laboratory abnormalities, %
Summary
- High SVR12 rates after 12 weeks of Glecaprevir + Pibrentasvir
with only 2 virologic failures in 50 DAA-experienced genotype 1 patients without cirrhosis
- Baseline NS3 and/or NS5A RAVs did not impact SVR12 (most patients (≥80%) had at least 1 baseline polymorphism in NS3 or NS5A)
- RBV did not increase SVR12 rate
- Good tolerability, with mild adverse events
- No grade 3 or 4 laboratory abnormalities
- No discontinuation due to adverse events