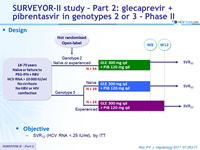
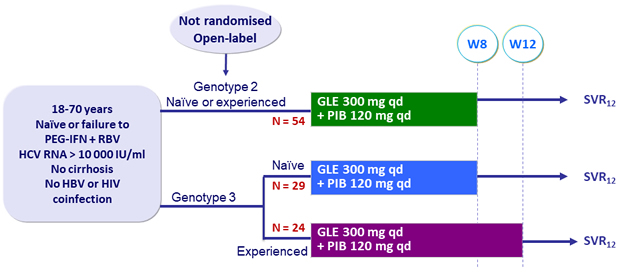
SURVEYOR-II study - Part 2: glecaprevir + pibrentasvir in genotypes 2 or 3 – Phase II
Kwo PY. J Hepatol 2017; 67 :263-71
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
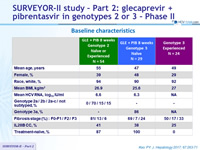
Genotype
2
3
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Design
Objective
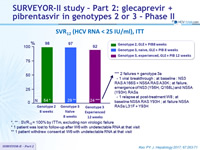
- SVR12 (HCV RNA < 25 IU /ml), by ITT
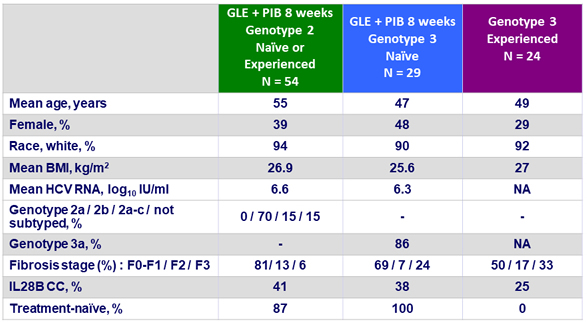
Baseline characteristics
SVR12 (HCV RNA < 25 IU /ml), ITT
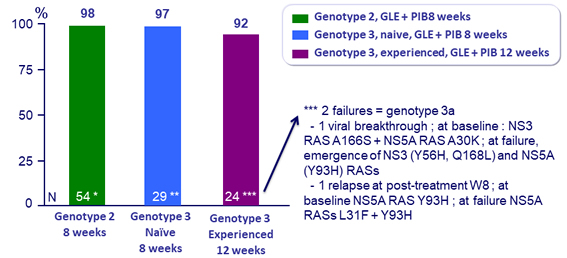
*, ** : SVR 12 = 100% by ITTm , excluding non virologic failure
* 1 patient was lost to follow-up after W6 with undetectable RNA at that visit
** 1 patient withdrew consent at W6 with undetectable RNA at that visit
Resistance analysis (population sequencing with 15% threshold)
- Baseline RAVs
- 58% of genotype 2 : NS3 only in 13% , NS5A only in 38%, NS3 + NS5A in 6%
- 46% of genotype 3 without cirrhosis, 38% of genotype 3 with cirrhosis
- No impact on SVR12
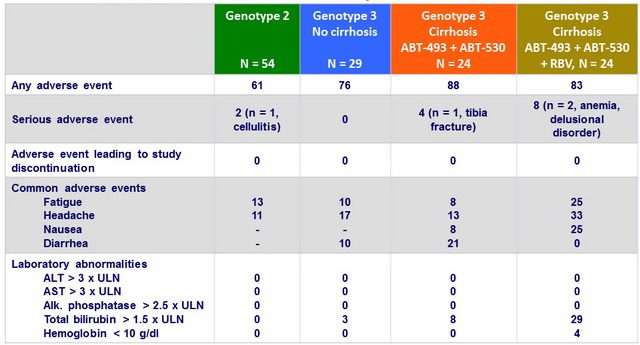
Adverse events and laboratory abnormalities , 8 weeks of GLE + PIB, %
Summary
- Very high SVR rates were achieved in HCV after 8 weeks of GLE 300 mg + PIB 120 mg qd
- In genotype 2 naïve or experienced and genotype 3 naïve patients without cirrhosis
- By ITTm, all patients achieved SVR12
- No impact on efficacy of baseline NS3 and/or NS5A RASs
- 92% SVR12 with 12 weeks once-daily GLE 120 mg + PIB 120 mg in treatment-experienced genotype 3-infected patients without cirrhosis
- Adverse events were mostly mild in severity