GALAXY study: SMV + SOF ± RBV in recurrent genotype 1 HCV infection post liver transplantation
O’Leary JG. Transplant International 2017; 30:196-208
Anti-HCV
Simeprevir
Sofosbuvir
Ribavirin
Simeprevir
Sofosbuvir
Ribavirin
Genotype
1
1
Cirrhosis
No
No
Special population
Liver transplantation
Liver transplantation
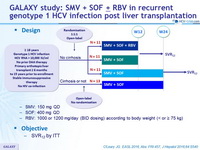
Design
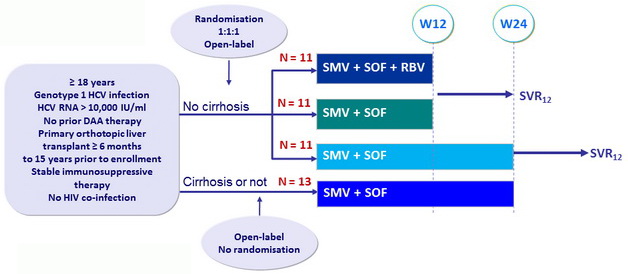
- SMV: 150 mg QD
- SOF: 400 mg QD
- RBV : 1000 or 1200 mg/day (BID dosing) according to body weight (< or = 75 kg)
Objective
- SVR12 by ITT
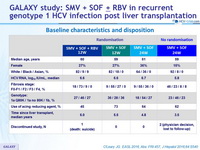
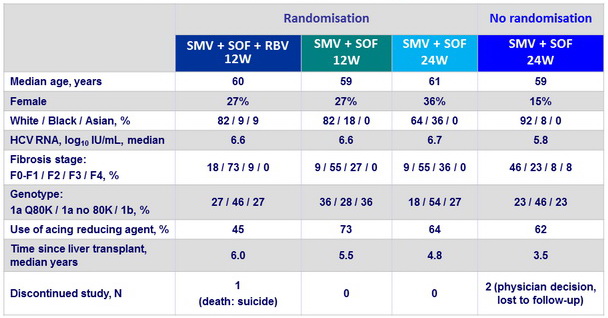
Baseline characteristics and disposition
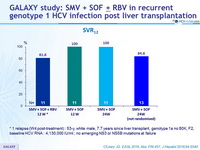
SVR12
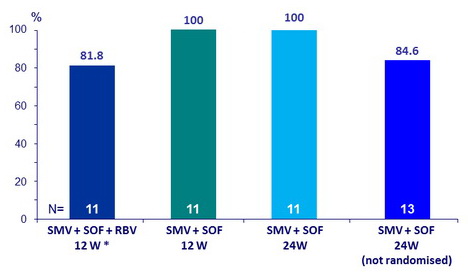
* 1 relapse (W4 post-treatment) : 53-y, white male, 7.7 years since liver transplant, genotype 1a no 80K, F2, baseline HCV RNA : 4,130,000 IU/ml ; no emerging NS3 or NS5B mutations at failure
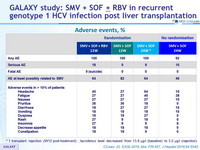
Adverse events, %
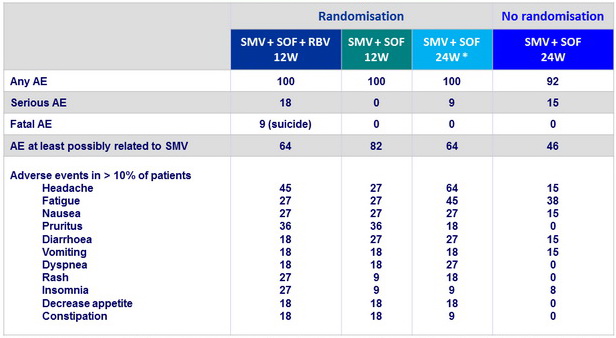
* 1 transplant rejection (W12 post-treatment) ; tacrolimus level decreased from 13.8 m g/l (baseline) to 3.2 m g/l (rejection)
Summary
- Treatment with SMV + SOF with or without RBV, for 12 weeks, or with SMV + SOF, for 24 weeks, was efficacious in liver transplant recipients with recurrent HCV genotype 1 infection
- Overall SVR12 of 91.3%
- 1 relapse occurred after 12 weeks of SMV + SOF + RBV
- Treatment was generally well tolerated
- 1 transplant rejection was observed, in the context of decreased tacrolimus plasma levels
- In conclusion, 12 weeks of SMV + SOF is an effective combination for liver transplant recipients without cirrhosis presenting recurrence of HCV genotype 1 infection