TRILOGY-3 Study: SOF/VEL/VOX ± RBV in DAA-experienced patients - Phase II
Lawitz E. Hepatology 2017; 65:1803-9
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
1
1
Treatment history
NS5A experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
Design
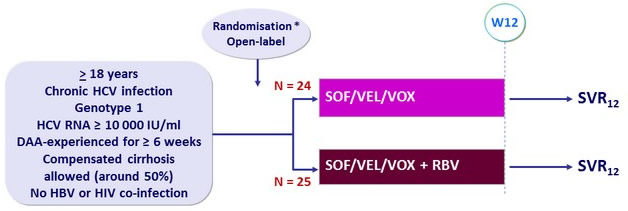
* Randomisation was stratified by cirrhosis (yes or no) and NS5A inhibitor experience (yes or no)
- SOF/VEL/VOX: 400/100/100 mg FDC qd
- BV : 1000 or 1200 mg/day, according to body weight
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT, with 95% CI
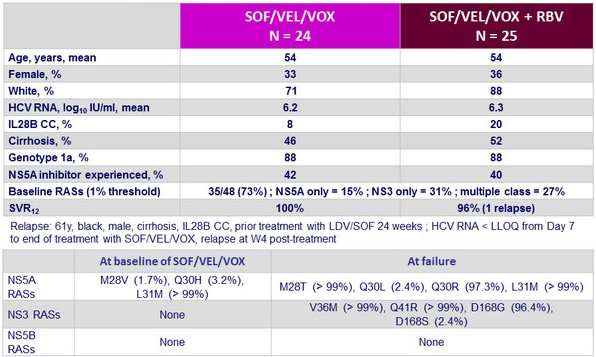
Baseline characteristics and outcome
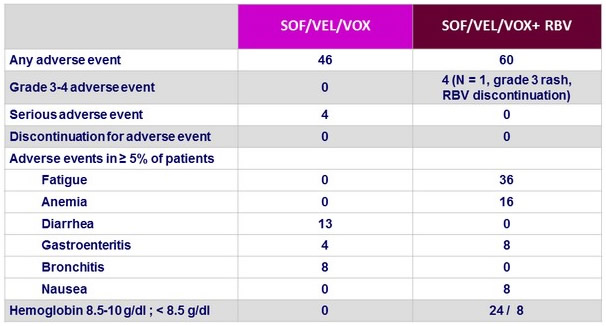
Adverse events and laboratory abnormalities %
Summary
- SOF/VEL /VOX
once daily ± RBV for 12 weeks achieved high SVR12 rate in HCV genotype 1-infected patients with prior DAA experience
- Addition of RBV did not enhance SVR
- Baseline RASs did not reduce SVR
- SVR12 = 13/13 (100%) if no baseline RASs vs 34/35 (97%) if baseline RASs
- The combination was generally well tolerated
- Adverse event profile was less good with RBV




