Retreatment study: SOF/VEL + RBV in prior NS5A failure-Phase II.
Gane EJ, Hepatology. 2017 ; 66 :1083-9
Anti-HCV
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
1
1a
2
3
1
1a
2
3
Treatment history
PI (NS3)-experienced
NS5A experienced
PI (NS3)-experienced
NS5A experienced
Cirrhosis
No
No
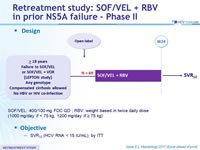
Design
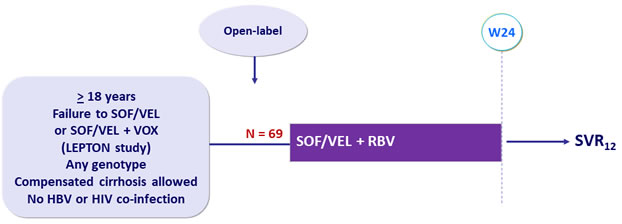
- SOF/VEL: 400/100 mg FDC QD ; RBV: weight based in twice daily dose (1000 mg/day if < 75 kg, 1200 mg/day if = 75 kg)
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT
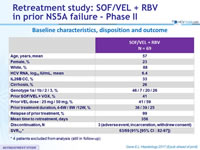
Baseline characteristics, disposition and outcome
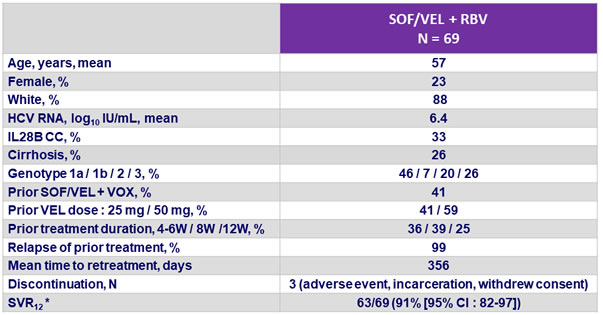
* 4 patients excluded from analysis (still in follow-up)
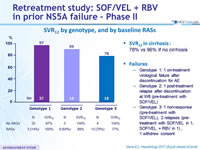
SRV12 by genotype, and by baseline RAVs
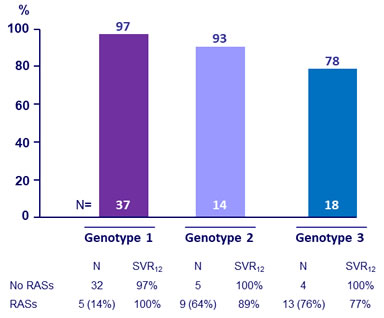
SRV12 in cirrhosis
- 78% vs 96% if no cirrhosis
Failures
- Genotype 1: 1 on-treatment virological failure after discontinuation for AE
- Genotype 2: 1 post-treatment relapse after discontinuation at W8 (pre-treatment with SOF/VEL)
- Genotype 3: 1 non-response ((pre-treatment with SOF/VEL), 2 relapses (pre-treatment with SOF/VEL in 1, SOF/VEL + RBV in 1), 1 withdrew consent
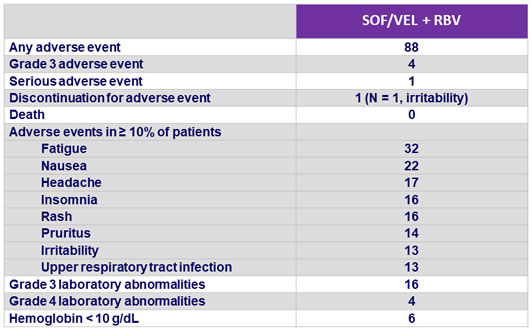
Adverse events and laboratory abnormalities, %
Summary
- SOF/VEL + RBV for 24 weeks resulted in high SVR12 rates in patients with genotype 1 or 2 who had failed prior SOF/VEL-containing regimens
- Genotype 1: no impact of RASs on SVR12
- Genotype 3: SVR12 of 100% in patients without NS5A RASs, but low SVR12 among those with NS5A RASs
- Good tolerability