LEPTON Study: SOF/VEL + GS-9857 genotype 1 or 3 Phase II
Gane EJ, Gastroenterology 2016; 151:448-456
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Genotype
1
3
1
3
Treatment history
Naive
PI (NS3)-experienced
NS5A experienced
Naive
PI (NS3)-experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
Design
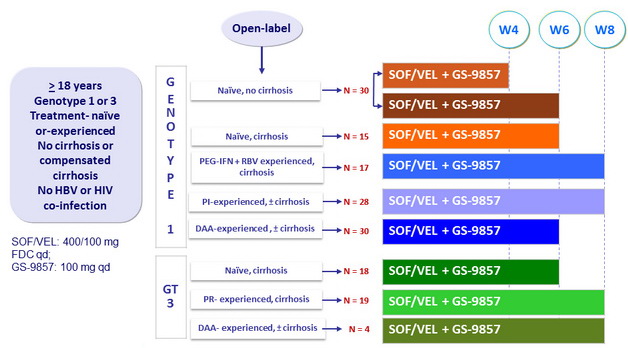
- SOF/VEL: 400/100 mg FDC qd ;
- GS-9857: 100 mg qd
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT
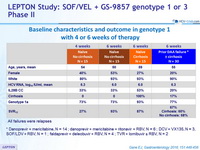
Baseline characteristics and outcome in genotype 1 with 4 or 6 weeks of therapy
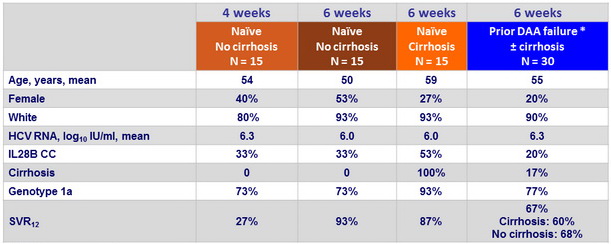
* Danoprevir + mericitabine , N = 14 ; danoprevir + mericitabine + ritonavir + RBV, N = 6 ; DCV + VX135, N = 3, SOF/LDV + RBV, N = 1 ; faldaprevir + deleobuvir + RBV, N = 4 ; TVR + lomibuvir ± RBV, N = 2
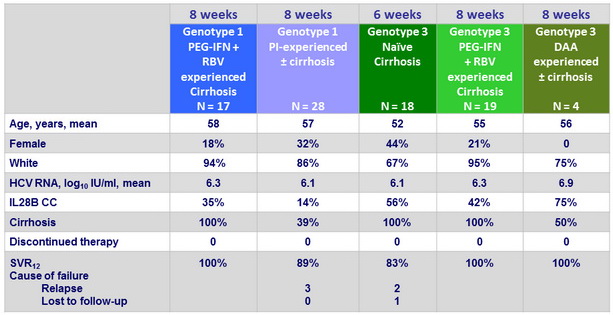
Baseline characteristics, disposition and outcome in genotype 1 with 8 weeks of therapy or in genotype 3
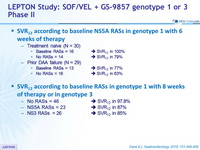
SVR12 according to baseline NS5A RASs in genotype 1 with 4 or 6 weeks of therapy
- Treatment naïve (N = 30)
- Baseline RASs = 16 >>> SVR12 in 100%
- No RASs = 14 >>> SVR12 in 79%
- Prior DAA failure (N = 29)
- Baseline RASs = 13 >>> SVR12 in 77%
- No RASs = 16 >>> SVR12 in 63%
SVR12 according to baseline RASs in genotype 1 with 8 weeks of therapy or in genotype 3
- No RASs = 46 >>> SVR12 in 97.8%
- NSRA RASs = 23 >>> SVR12 in 87%
- NS3 RASs = 26 >>> SVR12 in 85%
SVR12 according to prior failure
- Failure to PI-based therapy, N = 28
- Baseline NS3 RASs in 15/28 (54%) >>> SVR12 in 13/15 (87%)
- Failure to NS5A inhibitor (N = 7)
- Baseline NS3 RASs in 6/7 (86%) >>> SVR12 in 5/6 (83%)
Relapse, N = 28
- No emergence of RAS, N = 26
- No RAS at baseline and failure, N = 15
- Same RASs at baseline and failure, N = 6
- Baseline RASs but no RAS at failure, N = 5
- Emergence of RASs, N = 2
- Treatment-n aïve, 6 weeks of treatment: NS3 RAS V55A emerged at 2% of the viral population at the time of relapse
- PI-experienced, 8 weeks of treatment: NS5A RAS Y93H emerged at 2% of the viral population at the time of relapse in addition to the pree xisting NS3 RAS R155K
Adverse events and laboratory abnormalities, %
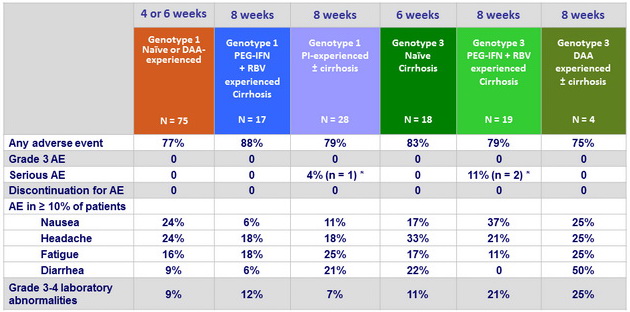
* Atrial fibrillation, hepatocellular carcinoma and bladder cancer, all unrelated to study drug
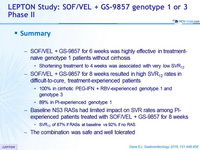
Summary
- SOF/VEL + GS-9857 for 6 weeks was highly effective in treatment-naïve genotype 1 patients without cirrhosis
- Shortening treatment to 4 weeks was associated with very low SVR12
- SOF/VEL + GS-9857 for 8 weeks resulted in high SVR12 rates in difficult-to-cure, treatment-experienced patients
- 100% in cirrhotic PEG-IFN + RBV-experienced genotype 1 and genotype 3
- 89% in PI-experienced genotype 1
- Baseline NS3 RASs had limited impact on SVR rates among PI-experienced patients treated with SOF/VEL + GS-9857 for 8 weeks
- SVR 12 of 87% if RASs at baseline vs 92% if no RAS
- The combination was safe and well tolerated