LDV/SOF in kidney transplant recipients
Colombo M, Ann Intern Med 2017; 166:109-117
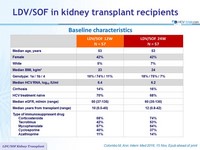
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1a
1b
4
1
1a
1b
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Special population
Chronic Kidney disease
Chronic Kidney disease
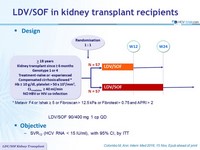
Design
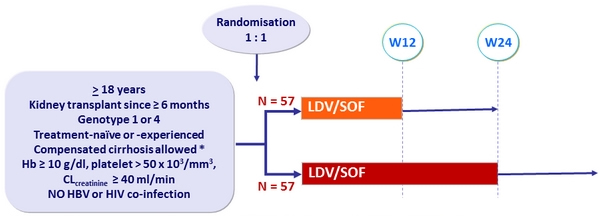
* Metavir F4 or Ishak = 5 or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2
- LDV/SOF 90/400 mg 1 cp QD
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 95% CI, by ITT
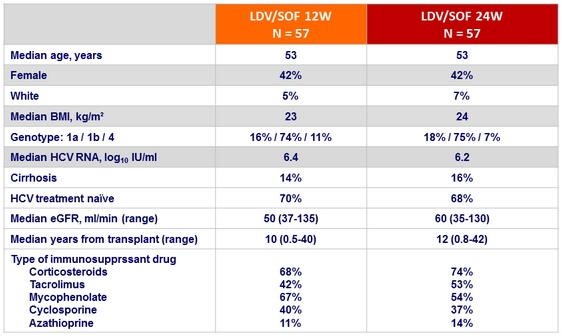
Baseline characteristics
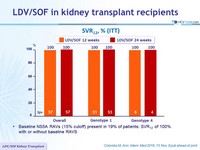
SRV12, % (ITT)
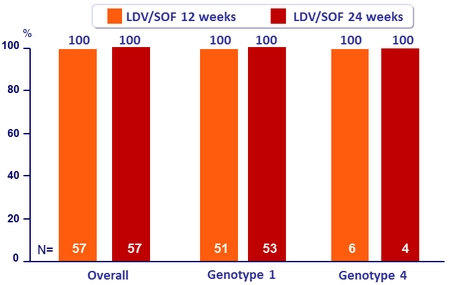
- Baseline NS5A RAVs (15% cutoff) present in 19% of patients: SVR12 of 100% with or without baseline RAVS
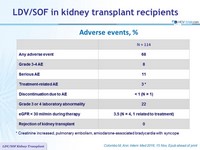
Adverse events, %
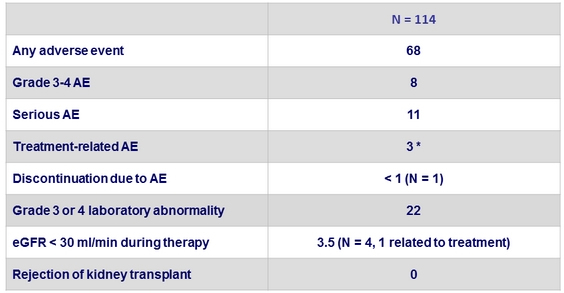
*
Creatinine increased, pulmonary embolism, amiodarone-associated bradycardia with syncope
Summary
- SOF/VEL for 12 weeks resulted in overall 100% SVR12 in genotype 1 or 4 HCV-infected kidney transplant patients
- With or without cirrhosis
- And/or history of prior treatment failure
- No need to extend therapy to 24 weeks
- Treatment was safe and well tolerated,
- With no clinically meaningful reduction in renal function
- median change in creatinine clearance [ eGFR by Cockcroft– Gault equation ] - 0.6 to - 3 ml/min during treatment and up to post-treatment W4
- With no clinically meaningful reduction in renal function