COMMIT Study: SMV + DCV in genotype 1b
Hezode C, Liver International 2017 ; 37:1304-13
Anti-HCV
Simeprevir
Daclatasvir
Simeprevir
Daclatasvir
Genotype
1b
1b
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
Design
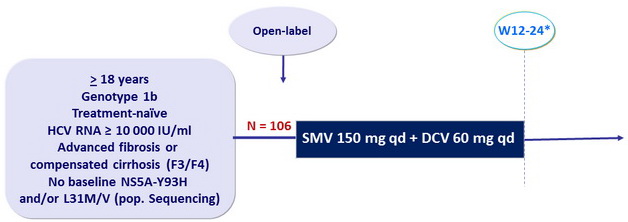
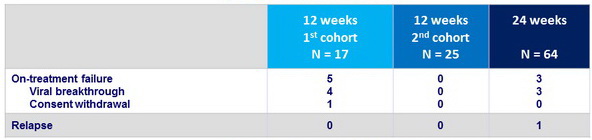
* 12 weeks in the first 17 patients ; due to observations of breakthrough, next 89 patients treated for 12 or 24 weeks based on patient wish and investigator discretion
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 95% CI, by ITT
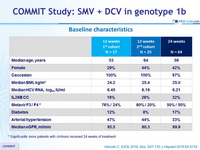
Baseline characteristics
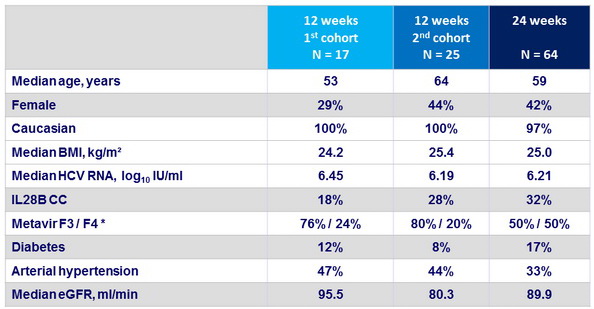
* Significantly more patients with cirrhosis received 24 weeks of treatment
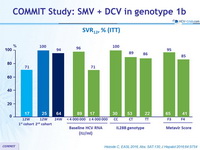
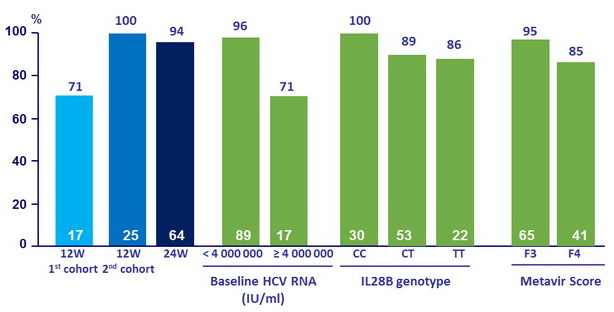
SVR12, % (ITT)
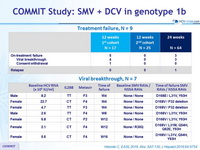
Treatment failure, N=9
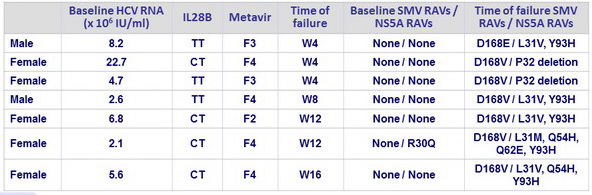
Viral breakthrough, N=7
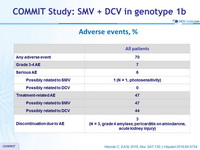
Adverse events, %
Summary
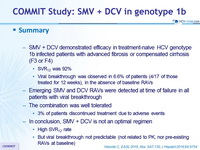
- SMV + DCV demonstrated efficacy in treatment-naïve HCV genotype 1b infected patients with advanced fibrosis or compensated cirrhosis (F3 or F4)
- SVR12 was 92%
- Viral breakthrough was observed in 6.6% of patients (4/17 of those treated for 12 weeks), in the absence of baseline RAVs
- Emerging SMV and DCV RAVs were detected at time of failure in all patients with viral breakthrough
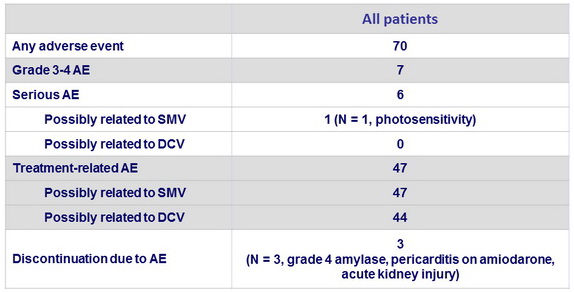
- The combination was well tolerated
- 3% of patients discontinued treatment due to adverse events
- In conclusion, SMV + DCV is not an optimal regimen
- High SVR12 rate
- But viral breakthrough not predictable (not related to PK, nor pre-existing RAVs at baseline)