GS-US-367-1168 Study: SOF/VEL + GS-9857
in genotype 1 - Phase II
Lawitz E, Gastroenterology 2016; 151:893-901
Anti-HCV
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Voxilaprevir (GS-9857)
Velpatasvir (GS-5816)
Sofosbuvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
NS5A experienced
PI (NS3)-experienced
SOF-experienced
Naive
NS5A experienced
PI (NS3)-experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
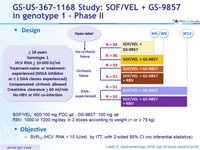
Design
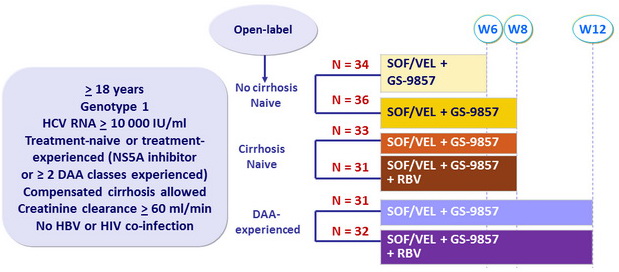
- SOF/VEL: 400/100 mg FDC qd ; GS-9857: 100 mg qd
- RBV: 1000 or 1200 mg/day in 2 doses according to weight (< or = 75 kg)
Objective
- SVR12 (HCV RNA < 15 IU/ml), by ITT, with 2-sided 95% CI (no inferential statistics)
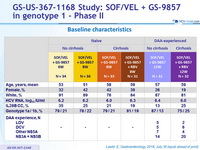
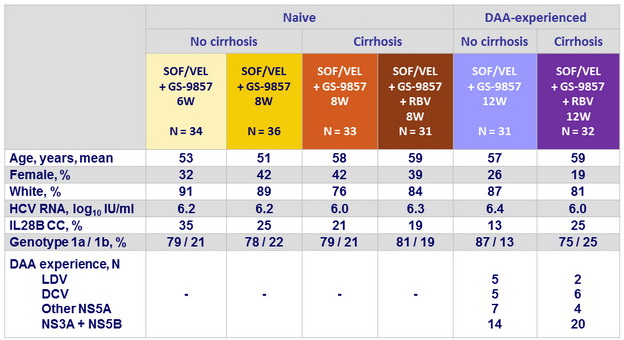
Baseline characteristics
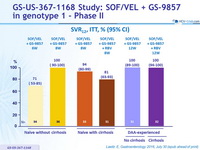
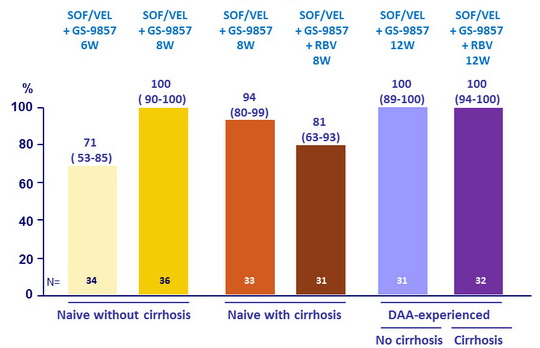
SRV12, ITT, % (95% CI)
Resistance analysis (1% deep sequencing)
- At baseline, presence of class RASs (NS3, NS5A or NS5B)
- 81/134 (60%) treatment-naïve patients
- 51/63 (81%) DDA-experienced patients
- 93% if NS5A-experienced vs 18% if not NS5A-experienced
- In treatment-naïve patients, 8 weeks of SOF/VEL + GS-9857 led to SVR 12 in 96% (25/26) and 97% (42/43) of patients without and with baseline RASs, respectively
- All DAA-experienced patients, regardless of the presence of single or multi-class RASs, achieved SVR12 following 12 weeks of SOF/VEL + GS-9857
- At relapse (sequencing data in 17/18):
- 10/17 (59%) had the same number or fewer RASs at the time of virologic relapse than at baseline
- 4/17(24%) had no RASs both at baseline and at virologic relapse
- Only 3 patients had treatment-emergent RASs, all in the NS3 gene and all at frequencies less than 2% of the viral population: V170T, Q41R + A156T, V36M
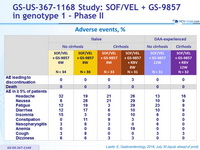
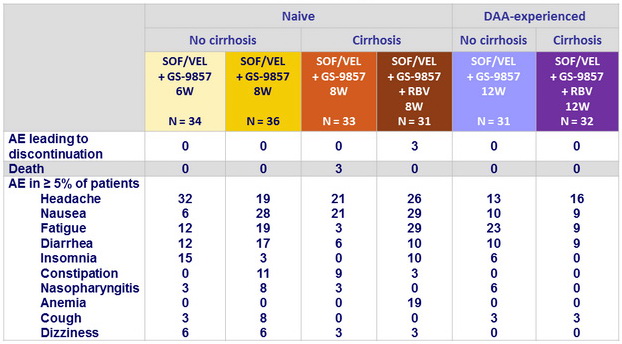
Adverse events, %
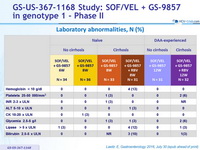
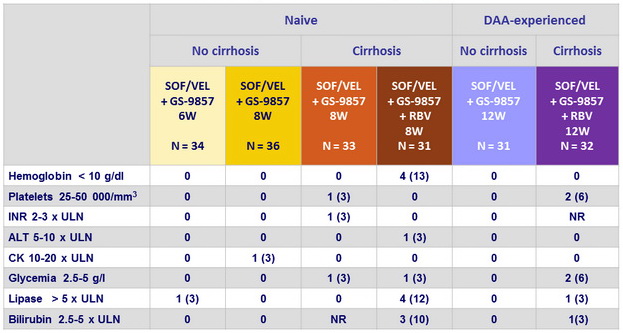
Laboratory abnormalities, N (%)
Summary
- In this phase 2 open-label trial, 8 weeks treatment with SOF/VEL + GS-9857 was safe and effective in treatment-naïve patients ; 12 weeks was safe and effective in patients previously treated with DAAs
- The combination was safe and effective in patients with or without compensated cirrhosis