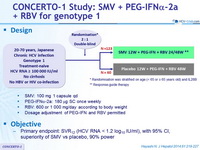
CONCERTO-1 Study: SMV + PEG-IFNα-2a + RBV for genotype 1
Hayashi N. J Hepatol 2014;61:219-227
Anti-HCV
Simeprevir
PEG-IFNα 2a
Ribavirin
Simeprevir
PEG-IFNα 2a
Ribavirin
Genotype
1b
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
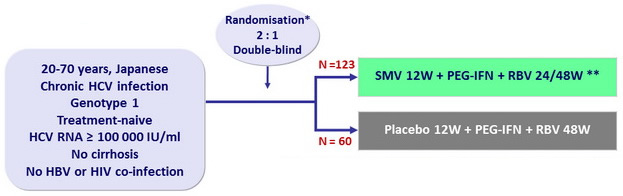
* Randomisation was stratified on age (< 65 or = 65 years old ) and IL28B
** Response -guide therapy
- SMV: 100 mg 1 capsule qd
- PEG-IFNα-2a: 180 m g SC once weekly
- RBV: 600 or 1 000 mg/day according to body weight
- Dosage adjustment of PEG-IFN and RBV permitted
Objective
- Primary endpoint: SVR12 (HCV RNA < 1.2 log10 IU/ml) , with 95% CI, superiority of SMV vs placebo, 90% power
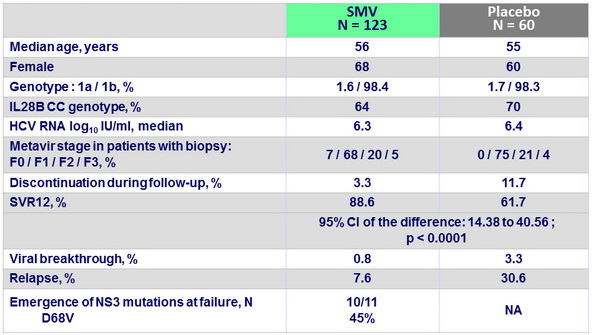
Baseline characteristics, patient disposition and outcome
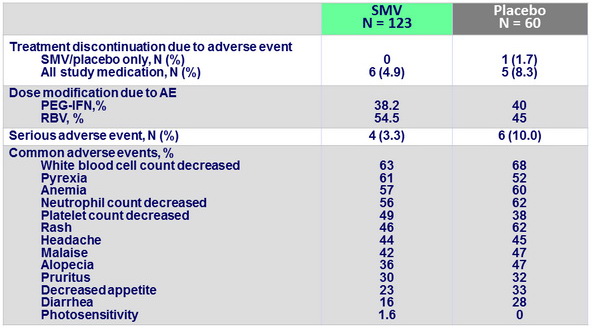
Adverse events, N or %
Summary
- In treatment-naive patients with HCV genotype 1 and high viral load, oral once-daily SMV in combination with PEG-IFN + RBV significantly improves SVR rates and shortens treatment duration in most patients
- 91.9% of SMV-treated patients met response-guided therapy criteria and completed treatment at W24; SVR12 in these patients was 92.0%
- Viral relapse occurred infrequently in SMV-treated patients, being observed in 7.6% of SMV-treated patients with undetectable plasma HCV RNA at end of treatment
- Emerging mutations were detected in most SMV-treated patients who experienced treatment failure, with D168V the most frequent emerging mutation
- SMV had a clinically favorable safety and tolerability profile in this patient population