CONCERTO-2 Study: SMV + PEG-IFNα-2a + RBV for genotype 1
Izumi N, J Gastroenterol 2014;49:941-53
Anti-HCV
Simeprevir
PEG-IFNα 2a
Ribavirin
Simeprevir
PEG-IFNα 2a
Ribavirin
Genotype
1b
1b
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
Design
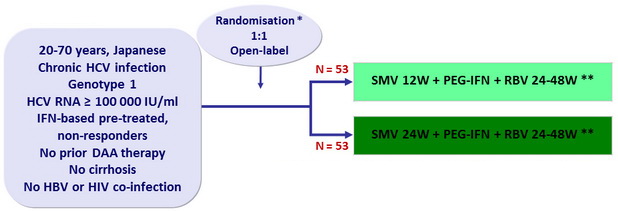
* Randomisation was stratified on age (< 65 or = 65 years old) and IL28B
** Response-guided therapy
- SMV: 100 mg 1 capsule qd
- PEG-IFNα-2a: 180 mg SC once weekly
- RBV: 600 or 1000 mg/day according to body weight
- Dosage adjustment of PEG-IFN and RBV permitted
Objective
- Primary endpoint: SVR12 (HCV RNA < 1.2 log10 IU/ml) , with 2-sided 95% CI, significant difference vs null hypothesis proportion = 14% of success, 90% power
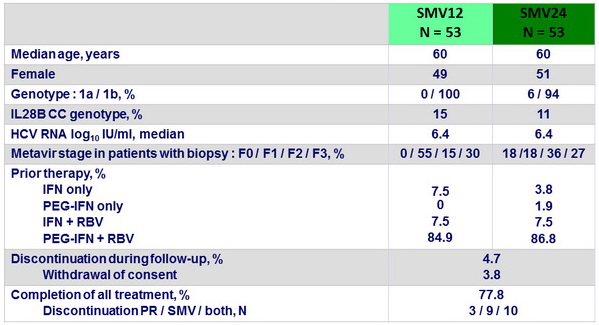
Baseline characteristics, and disposition
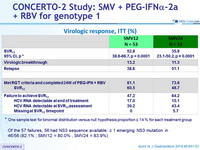
Virologic response, ITT (%)
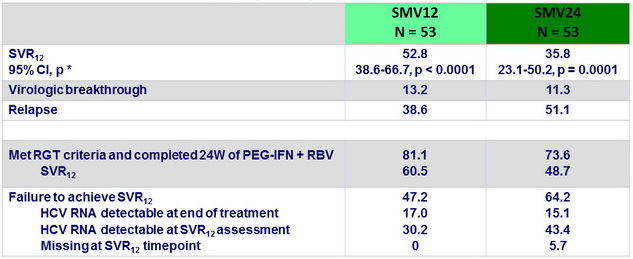
* One sample test for binomial distribution versus null hypothesis proportion = 14 % for each treatment group
- Of the 57 failures, 56 had NS3 sequence available: = 1 emerging NS3 mutation in 46/56 (82.1% ; SMV12 = 80.0% , SMV24 = 83.9%)
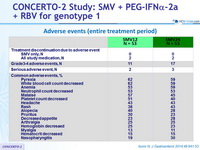
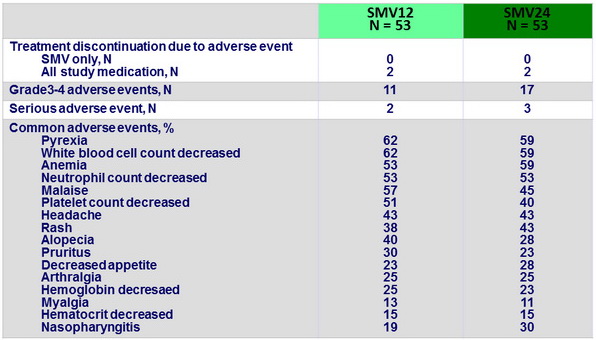
Adverse events (entire treatment period)
Summary
- In treatment-experienced patients with HCV genotype 1 infection who failed to respond to previous IFN-based therapy, re-treatment with 12 weeks of oral SMV QD in combination with PEG-IFN +RBV achieves high SVR rate
- This study was not able to determine whether there is an additional efficacy benefit by prolonging SMV therapy beyond 12 weeks
- Limitation:
- prior partial response versus null-response status to previous IFN-based therapy was not clearly documented
- SMV was generally well tolerated the incidences of serious adverse events or grade 3/4 rash or anemia were low, as were the rates of treatment discontinuations due to these adverse events