CONCERTO-3 Study: SMV + PEG-IFNα-2a + RBV for genotype 1
Izumi N, J Gastroenterol 2014;49:941-53
Anti-HCV
Simeprevir
PEG-IFNα 2a
Ribavirin
Simeprevir
PEG-IFNα 2a
Ribavirin
Genotype
1b
1b
Treatment history
IFN-Experienced
IFN-Experienced
Cirrhosis
No
No
Design
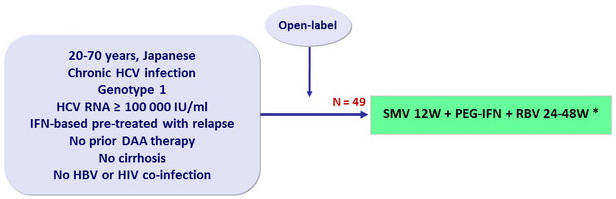
* Response-guided therapy
- SMV: 100 mg 1 capsule qd
- PEG-IFNα-2a: 180 mg SC once weekly
- RBV: 600 or 1000 mg/day according to body weight
- Dosage adjustment of PEG-IFN and RBV permitted
Objective
- Primary efficacy endpoint: SVR12 (HCV RNA < 1.2 log10 IU/ml) , with 2-sided 95% CI, significant difference vs null hypothesis proportion = 50% of success, 90% power
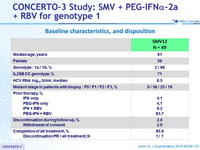
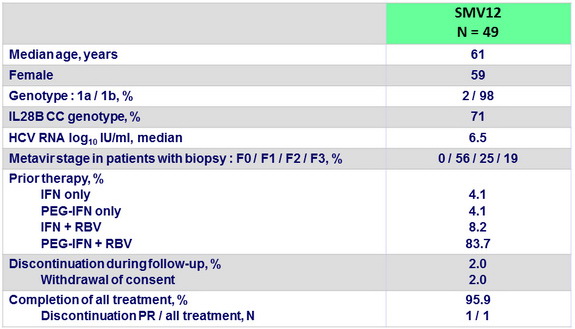
Baseline characteristics, and disposition
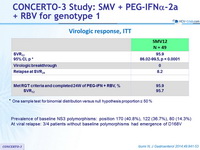
Virologic response, ITT
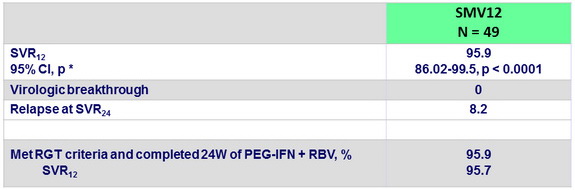
* One sample test for binomial distribution versus null hypothesis proportion = 50 %
- Prevalence of baseline NS3 polymorphisms: position 170 (40.8%), 122 (36.7%), 80 (14.3%)
- At viral relapse: 3/4 patients without baseline polymorphisms had emergence of D168V
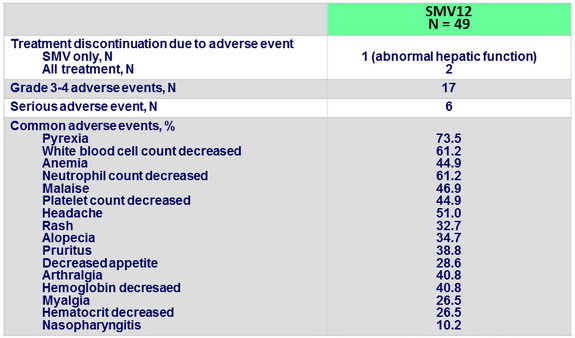
Adverse events (entire treatment period)
Summary
- In treatment-experienced patients with HCV genotype 1 infection who relapsed after previous IFN-based therapy, re-treatment with 12 weeks of oral SMV QD in combination with PEG-IFN + RBV achieves high SVR rate
- SMV was generally well tolerated the incidences of serious adverse events or grade 3/4 rash or anemia were low, as were the rates of treatment discontinuations due to these adverse events