CONCERTO-4 Study: SMV + PEG-IFNα-2b + RBV for genotype 1
Kumada H. Hepatol Research 2015;45:501-13
Anti-HCV
Simeprevir
PEG-IFNα 2b
Ribavirin
Ledipasvir
Sofosbuvir
Simeprevir
PEG-IFNα 2b
Ribavirin
Ledipasvir
Sofosbuvir
Genotype
1b
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
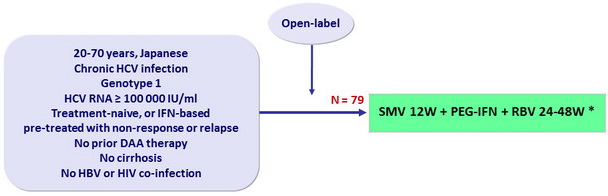
* Response-guided therapy in naive and relapsers ; W48 in non-responders
- SMV: 100 mg 1 capsule qd
- PEG-IFNα-2b: 1.5 µg/kg SC once weekly
- RBV: 600 or 1000 mg/day according to body weight
- Dosage adjustment of PEG-IFN and RBV permitted
Objective
- Primary efficacy endpoint: SVR12 (undetectable HCV RNA), with 95% CI
- Safety: 70 patients sufficient to detect a 97% probability of detecting an adverse event of special interest with = 5% incidence
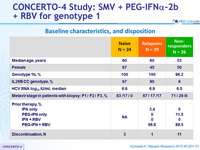
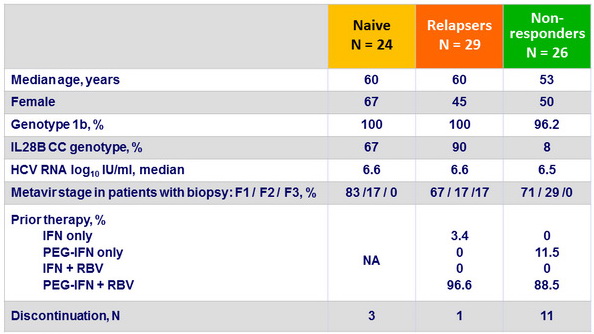
Baseline characteristics, and disposition
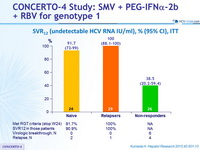
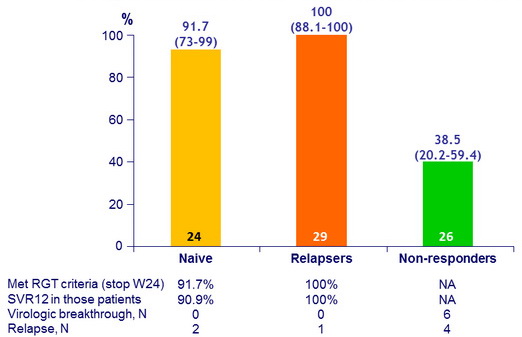
SVR12 (undetectable HCV RNA IU/ml), % (95% CI), ITT
Emerging mutations in treatment failure
- Sequencing analysis of NS3: 17/18 failures
(naive = 2, relapser = 1, non-responders = 14)
- Emerging mutations: 16/17
- Most frequent emerging mutations
- D168V, N = 8
- Q80R + D168E, N = 3
- D168E, N = 2
- R155K, N = 1
- D168T, N = 1
- Q80K + D168E, N = 1
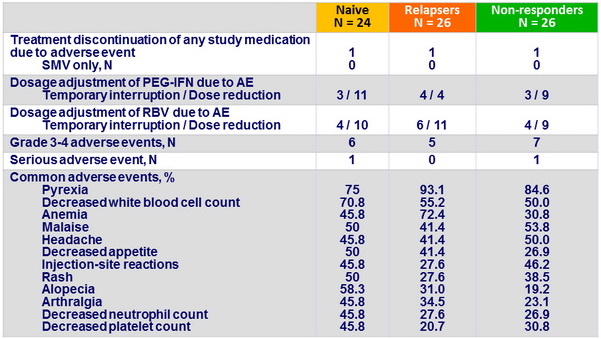
Adverse events (entire treatment period)
Summary
- Treatment with SMV 100 mg qd for 12 weeks in combination with PEG IFN-a-2b + RBV (for 24 or 48 weeks) demonstrated potent antiviral activity and high rates of SVR12 in patients who were treatment-naive or had previously relapsed after IFN-based therapy, with most patients having a shorter treatment duration (24 weeks)
- Antiviral activity was also demonstrated in some patients who had failed to respond to prior IFN-based therapy
- SMV was well tolerated in all patients
- No discontinuation of SMV for adverse event
- Only 1 case of serious adverse event considered related to SMV (hyperbilirubinemia)
- Limitation: small sample size for the 3 populations (79 patients in total)