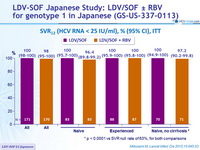
LDV-SOF Japanese Study: LDV/SOF ± RBV for genotype 1 in Japanese (GS-US-337-0113)
Mikozami M, Lancet Infect Dis 2015;15:645-53
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
1b
1b
Treatment history
Naive
IFN-Experienced
PI (NS3)-experienced
Naive
IFN-Experienced
PI (NS3)-experienced
Cirrhosis
Yes
No
Yes
No
Design
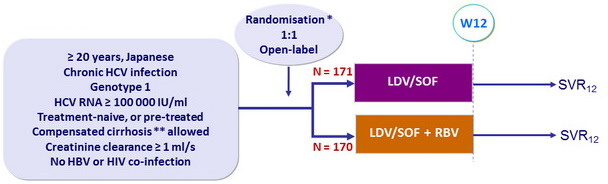
* Randomisation of naive patients stratified on cirrhosis (yes or no) ; Randomisation of pre-treated patients stratified on cirrhosis and prior response to previous therapy (relapse, non-response, IFN-intolerance)
** Liver biopsy ( Metavir F4 or Ishak = 5), or Fibroscan > 12.5 kPa
- LDV/SOF 90/400 mg 1 pill qd
- RBV (divided dose): 600 mg/d if < 60 kg, 800 mg/d if > 60 to = 80 kg, 1000 mg/d if = 80 kg
Objective
- Primary endpoint: SVR12 (HCV RNA < 25 IU /ml), with 2-sided 95% CI
- For naive patients without cirrhosis, SVR > 23% of the adjusted historical SVR null rate of 63%, 90% power; for other patients, no statistical hypothesis testing
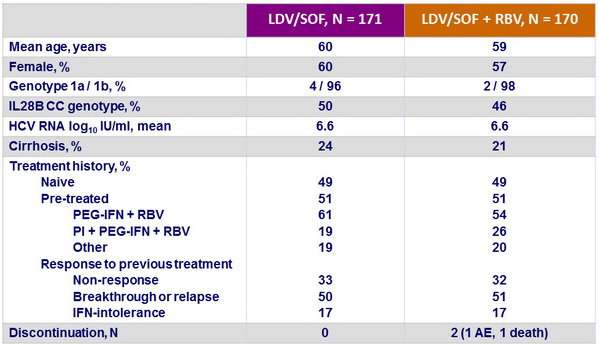
Baseline characteristics, and disposition
SVR12 (HCV RNA < 25 IU/ml) , % (95% CI), ITT
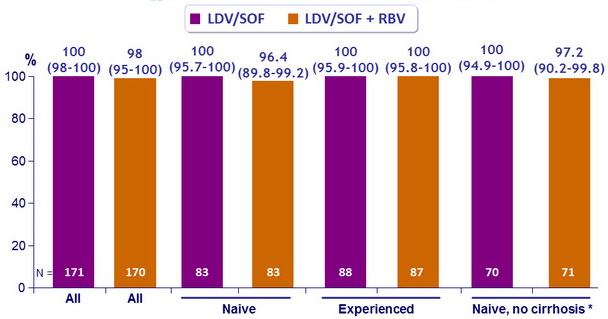
* p < 0.0001 vs SVR null rate of 63%, for both comparisons
Patients with NS5A RAV at baseline, N = 76
- SVR12 in 42/42 on LDV/SOF
- SVR12 in 33/34 on LDV/SOF + RBV
1 virological failure
- Treatment-naive, genotype 1b, 55-year-old woman without cirrhosis who was receiving LDV/DOF + RBV, relapse by post-treatment W4 after completion of treatment. Adherence rates > 99% for both LDV/SOF and RBV (800 mg daily)
- Baseline NS5A RAV: Y93H (> 99%) NS5A. No other NS5A RAVs were detected at post-treatment W4
No NS5B RAVs and no treatment-emergent variants were detected in any patient at any timepoint tested
Adverse events
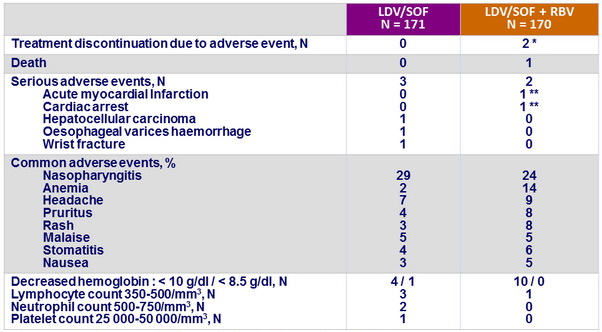
* drug eruption, N = 1, morbilliform rash, N = 1 ;
** related to study drug
Summary
- In this trial, 12 weeks of treatment with the fixed-dose combination of LDV/SOF without RBV was well tolerated and resulted in SVR12 in all 171 patients (100%) treated, including patients typically difficult to treat, including those with cirrhosis, or baseline NS5A RAVs, and those who had previously not responded well to other HCV treatment regimens, including PI-based therapies
- The addition of RBV to LDV/SOF let to a SVR 12 of 97%, and was associated with an increased number of patients who had adverse events
- Limitations of the study
- Open-label design
- Absence of an active comparator