LDV/SOF ± RBV in genotype 3 or 6 – Phase 2
Gane EJ, Gastroenterology 2015;149:1454-61
Anti-HCV
Ledipasvir
Sofosbuvir
Ribavirin
Ledipasvir
Sofosbuvir
Ribavirin
Genotype
3
6
3
6
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
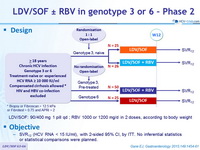
Design
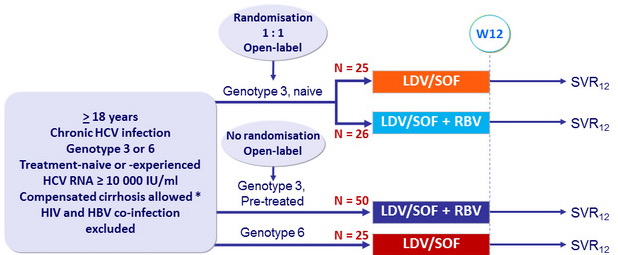
* Biopsy or Fibroscan > 12.5 kPa or Fibrotest > 0.75 and APRI > 2
- LDV/SOF: 90/400 mg 1 pill qd ; RBV 1000 or 1200 mg/d in 2 doses, according to body weight
Objective
- SVR12 (HCV RNA < 15 IU /ml), with 2-sided 95% CI, by ITT. No inferential statistics or statistical comparisons were planned.
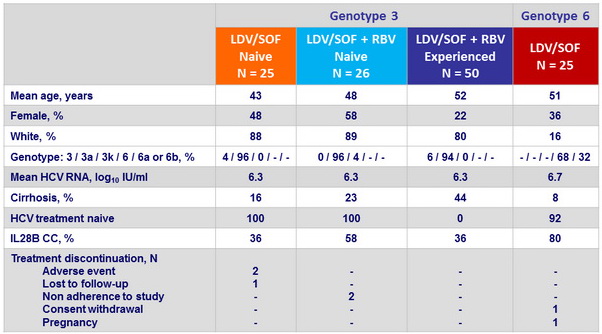
Baseline characteristics
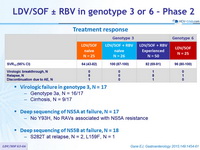
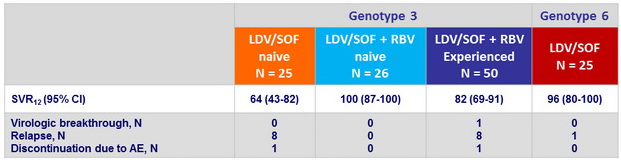
Treatment response
- Virologic failure in genotype 3, N = 17
- Genotype 3a, N = 16/17
- Cirrhosis, N = 9/17
- Deep sequencing of NS5A at failure, N = 17
- No Y93H, No RAVs associated with NS5A resistance
- Deep sequencing of NS5B at failure, N = 18
- S282T at relapse, N = 2, L159F, N = 1
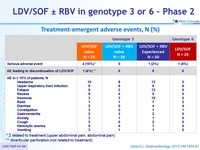
Treatment-emergent adverse events, N (%)
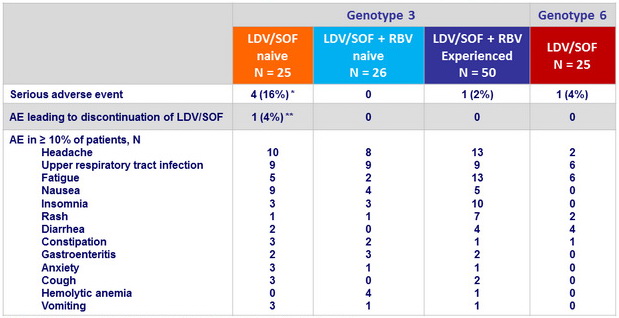
* 2 related to treatment (upper abdominal pain, abdominal pain)
** diverticular perforation (not related to treatment)
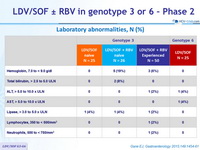
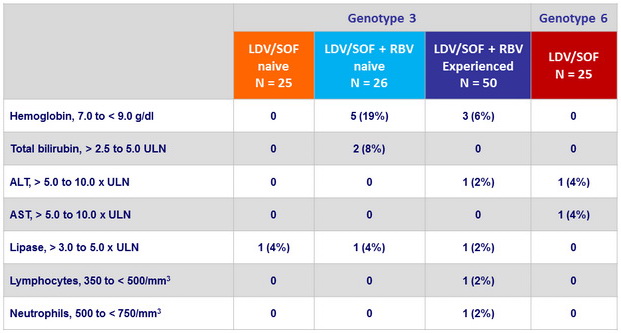
Laboratory abnormalities, N (%)
Summary
- In this open-label, phase 2 study, all 26 (100%) treatment-naive patients with genotype 3 HCV who were randomized to receive 12 weeks of LDV/SOF + RBV achieved SVR12 as compared with only 16 of 25 (64%) patients who received
12 weeks of LDV/SOF alone - The SVR12 rate was 82% in genotype 3 treatment-experienced patients receiving 12 weeks of LDV/SOF + RBV
- The SVR12 rate was 96% in genotype 6 patients receiving 12 weeks of LDV/SOF
- At virologic failure
- No emergence of NS5A RAVs
- Emergence of S282T in 2 patients and L159F in 1
- Most common adverse events were headache, upper respiratory infection, and fatigue
- Limitation
- Small size of treatment arms
- Lack of control group