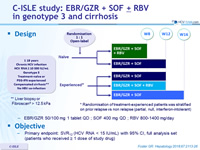
C-ISLE study: EBR/GZR + SOF ± RBV in genotype 3 and cirrhosis
Foster GR. Hepatology 2018;67:2113-26
Anti-HCV
Grazoprevir
Elbasvir
Sofosbuvir
Ribavirin
Grazoprevir
Elbasvir
Sofosbuvir
Ribavirin
Genotype
3
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
Yes
Design
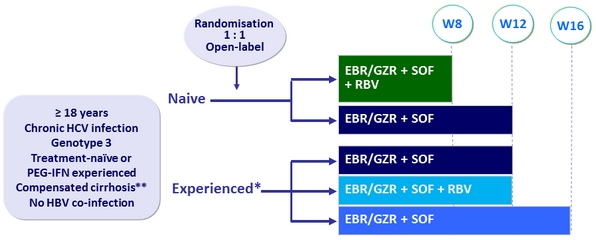
* Randomisation of treatment-experienced patients was stratified on prior relapse vs non relapse (partial, null, interferon-intolerant)
** Liver biopsy or Fibroscan® > 12.5 kPa
Objective
- Primary endpoint: SVR12 (HCV RNA < 15 IU/mL) with 95% CI, full analysis set (patients who received = 1 dose of study drug)
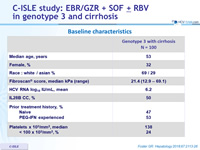
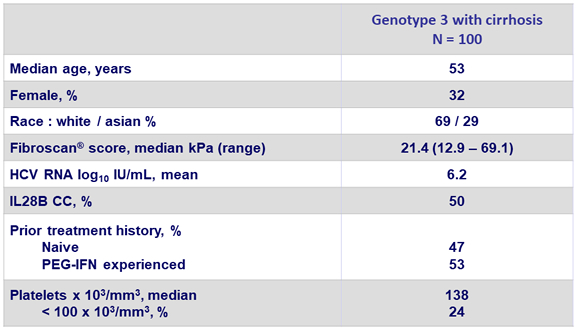
Baseline characteristics
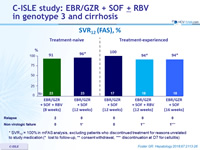
SVR12 (FAS), %
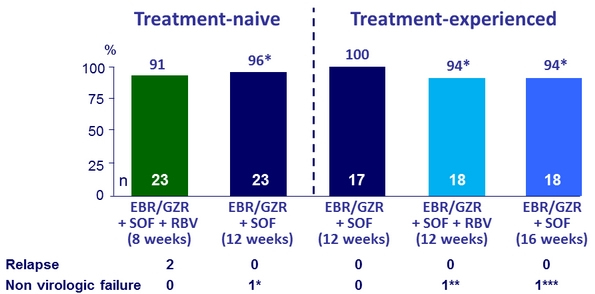
* SVR12 = 100% in mFAS analysis, excluding patients who discontinued treatment for reasons unrelated to study medication (* lost to follow-up, ** consent withdrawal, *** discontinuation at D7 for cellulitis)
Resistance data
- SVR12 according to baseline NS5A RASs (15% sensitivity threshold): NS5A polymorphisms at positions 24, 28, 30, 31, 32, 38, 58, 62, 92, or 93)
- 98% (49/50) if RASs
- 98% (46/47) if no RASs
- If Y93H present (N = 4): SVR12 = 0/1 (relapse) if 8 weeks treatment ; 3/3 (100%) if > 8 weeks treatment
2 relapses
- Both patients received EBR/GZR/SOF + RBV for 8 weeks
- Both were treatment-naïve
- In 1 patient : No NS5A RASs at baseline or failure
- In 1 patient : NS5A RASs at baseline and failure : Y93H, P58, S62T
Adverse events
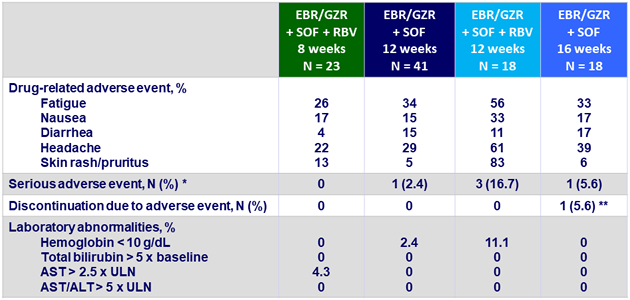
* Lung infection, creatinine increased, chest pain, opiate overdose, and cellulitis
** 1 patient discontinued treatment at Day 7 due to cellulitis
Summary
- High efficacy was demonstrated in treatment-naive and
treatment-experienced cirrhotic HCV genotype 3-infected patients
- There were no virologic failures among patients receiving EBR/GZR + SOF ± RBV for 12 or 16 weeks
- Treatment duration longer than 12 weeks is not needed
- Ribavirin is not needed
- High efficacy was seen regardless of
- presence of baseline NS5A RASs
- or patient characteristics
- Generally safe and well tolerated