C-WORTHY Study part B: grazoprevir + elbasvir ± ribavirin, 12 weeks vs 18 weeks in genotype 1
Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial
Lawitz E. Lancet 2015;385:1075-86
Anti-HCV
Grazoprevir
Elbasvir
Ribavirin
Grazoprevir
Elbasvir
Ribavirin
Genotype
1
1a
1b
1
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
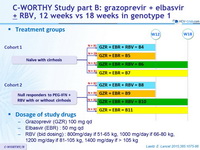
Design
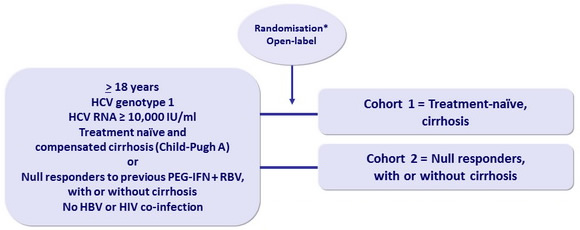
* Randomisation stratified on genotype (1a or non-1a) ; cohort 2 also stratified on cirrhosis (presence or absence).
Patients and investigators were masked to the duration of therapy until W12, but not to RBV allocation
Primary efficacy endpoint
- SVR12 (HCV RNA < 25 IU /ml), with 2-sided 95% CI, comparison between groups (intention to treat analysis)
Treatment groups
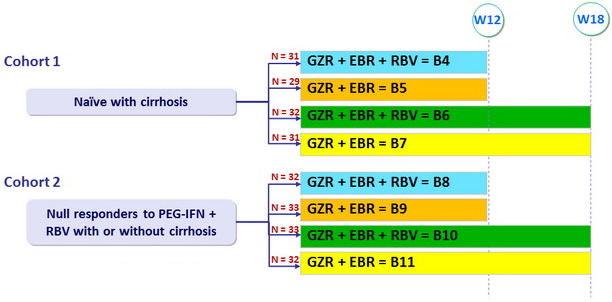
Dosage of study drugs
- Grazoprevir (GZR) 100 mg qd
- Elbasvir (EBR) : 50 mg qd
- RBV (bid dosing) : 800mg/day if 51-65 kg, 1000 mg/day if 66-80 kg, 1200 mg/day if 81-105 kg, 1400 mg/day if > 105 kg
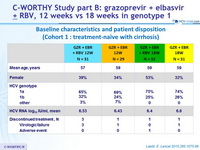
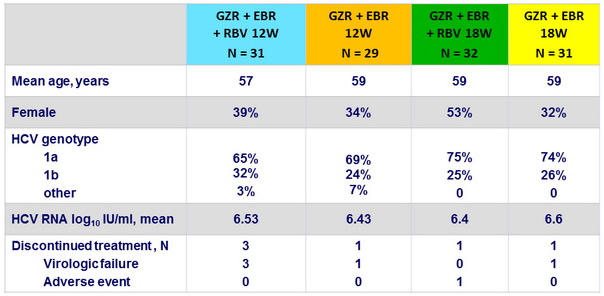
Baseline characteristics and patient disposition (Cohort 1 : treatment-naïve with cirrhosis)
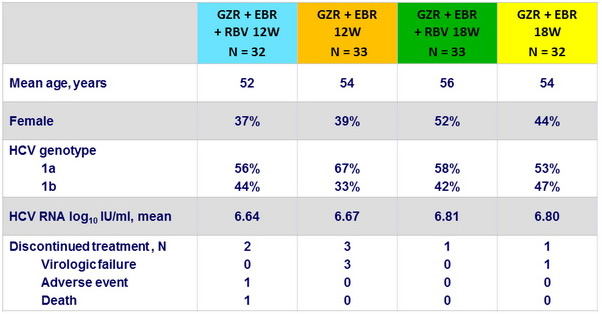
Baseline characteristics and patient disposition (Cohort 2 : null responders with or without cirrhosis)
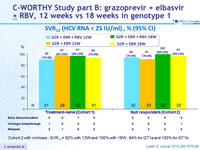
SVR12 (HCV RNA < 25 IU /ml), % (95% CI)
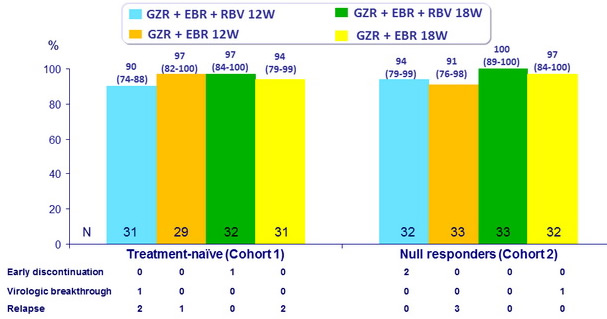
Cohort 2 with cirrhosis : SVR12 = 92% with 12W and 100% with 18W ; 94% for GT1a and 100% for GT1b
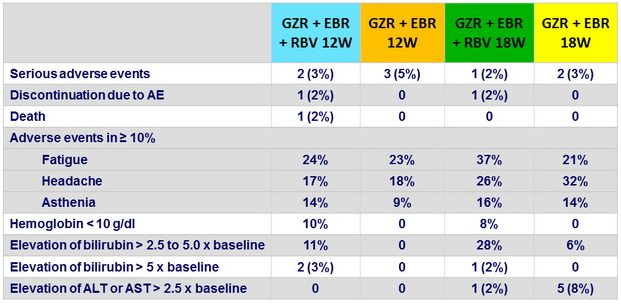
Adverse events and laboratory abnormalities, N (%)
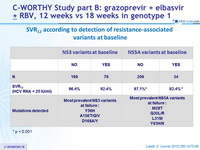
SVR12 according to detection of resistance-associated variants at baseline
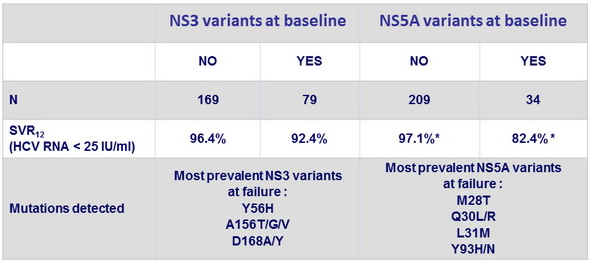
* p < 0.001
Summary
- In this phase II study, oral treatment with grazoprevir and elbasvir, with or without RBV, in HCV genotype 1-infected patients that are difficult to cure with HCV therapy (patients with well compensated cirrhosis and null responders with or without well compensated cirrhosis), high rates of SVR12 were shown across all groups, irrespective of the addition of RBV or extension of treatment duration from 12 to 18 weeks
- 12 weeks of GZR + EBR without RBV achieved SVR12 of
- 97% in previously untreated patients with cirrhosis,
- 91% in null responder patients with or without cirrhosis,
- 92% in null responder patients with cirrhosis
- The rate of virologic failure with GZR + EBR with or without RBV was low (4%)
- Similar efficacy was seen in patients with genotype 1a and 1b
- Patients with NS5A baseline resistance-associated variants had lower SVR12
- Treatment-emergent, clinically significant, adverse events were infrequent
- No discontinuation for AE in the groups without RBV
- 12 weeks of GZR + EBR without RBV achieved SVR12 of