ENDURANCE-2 Study: glecaprevir/pibrentasvir in genotype 2 without cirrhosis
Asselah T. Clin Gastroenterol Hepatol 2017 ; Sept 22 (Epub)
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
2
2
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Design
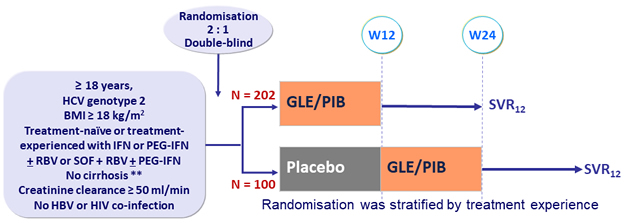
* Fibroscan® < 12.5 kPa or FibroTest® ≤ 0.48 + APRI < 1
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA < 15 IU/mL ): in SOF-naïve patients, non-inferiority by ITT, with lower margin of the 2-sided 95% CI > 89% ; superiority if lower margin of the 2-sided 95% CI > 95% (reference : historical rate of 95% of SVR 12 (12 weeks of SOF + RBV))
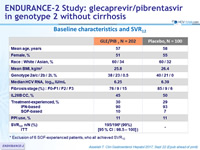
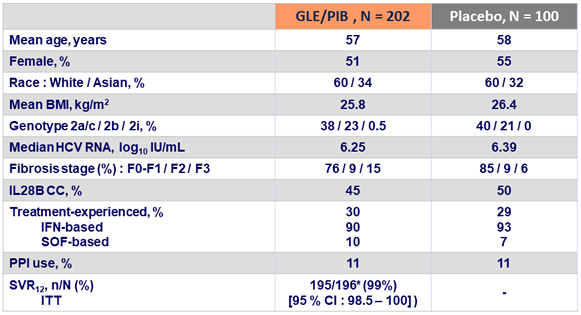
Baseline characteristics and SVR12
*
Exclusion of 6 SOF-experienced patients, who all achieved SVR12
Adverse events and laboratory abnormalities, N (%)
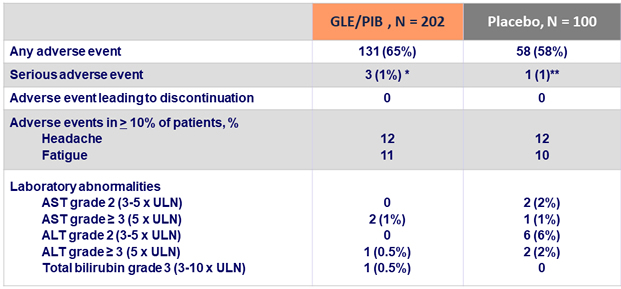
* One patient each experienced a broken ankle, hemorrhoids, and a bile duct stone; all were unrelated to treatment
** Rheumatoid arthritis, unrelated to treatmen
Summary
- 99% of patients with genotype 2 infection treated with GLE/PIB for 12 weeks achieved SVR12, with no virologic failures
- The primary and secondary endpoints were achieved: SVR12 rate achieved with GLE/PIB treatment was superior to the 95% historical SVR12 rate of SOF + RBV
- GLE/PIB was well tolerated:
- There were no discontinuations due to adverse event
- There were no serious adverse event related to GLE/PIB
- GLE/PIB demonstrated a safety profile similar to that observed in patients receiving placebo
- Achievement of SVR12 was not impacted by treatment experience or any other baseline factor