EXPEDITION-IV Study: glecaprevir/pibrentasvir in patients with renal impairment
Gane E. NEJM 2017 ; 377 :1448-55
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
2
3
4
1
2
3
4
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Special population
Chronic Kidney disease
Chronic Kidney disease
Design
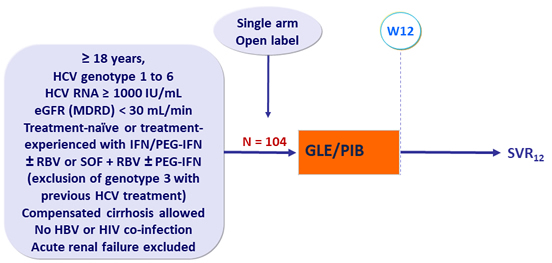
- GLE/PIB : 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA < 15 IU/ml), with 2-sided 95% CI, by ITT
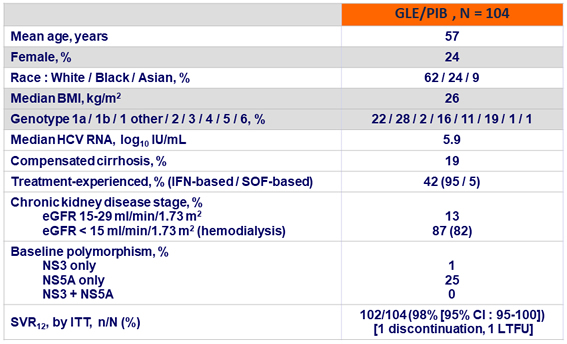
Baseline characteristics and SVR12
Adverse events and laboratory abnormalities, %
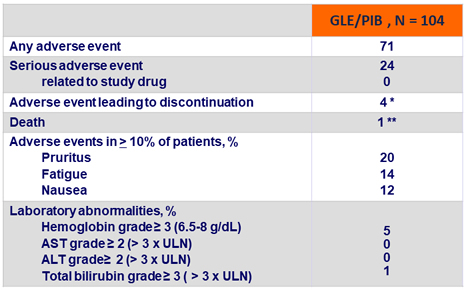
*
Diarrhea ; pruritus ; pulmonary edema, hypertensive cardiomyopathy with congestive failure ; hypertensive crisis
**
Serious adverse event of cerebral hemorrhage, not related to study drug, at post-treatment W2
Summary
- GLE/PIB (300 mg/120 mg QD) achieved high efficacy in patients with stage 4 or 5 chronic kidney disease and HCV infection
- 98% ITT SVR12 rate across all major HCV genotypes
- No virologic failures
- GLE/PIB was well tolerated with a favorable safety profile
in this difficult-to-treat population:
- No drug-related serious adverse event
- No grade ≥ 2 laboratory abnormalities in ALT or AST




