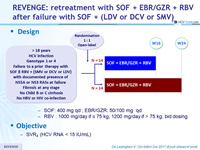
REVENGE: retreatment with SOF + EBR + GZR + RBV after failure with SOF + (LDV or DCV or SMV)
De Ledinghen V. Clin Infect Dis 2017, Oct 25 (Epub)
Anti-HCV
Grazoprevir
Elbasvir
Sofosbuvir
Ribavirin
Grazoprevir
Elbasvir
Sofosbuvir
Ribavirin
Genotype
1a
1b
4
1a
1b
4
Treatment history
NS5A experienced
SOF-experienced
NS5A experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
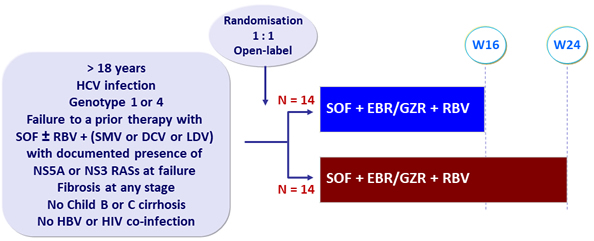
Design
- SOF: 400 mg qd ; EBR/GZR: 50/100 mg qd
- RBV : 1000 mg/day if = 75 kg, 1200 mg/day if > 75 kg, bid dosing
Objective
- SVR4 (HCV RNA < 15 IU/ml)
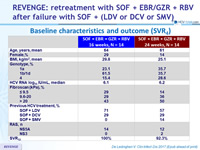
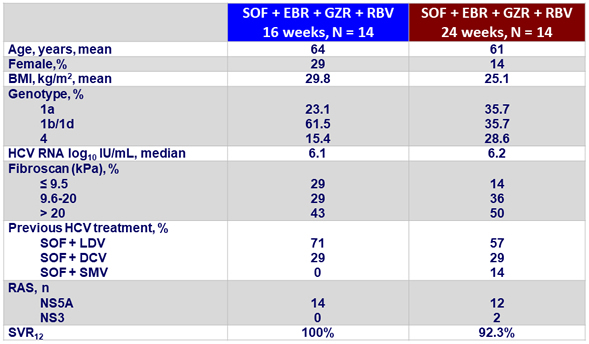
Baseline characteristics and outcome (SVR4)
Safety
- Serious adverse events, N = 9, in 7 patients (28%) :
- Right hypochondrium pain, dermo-hypodermitis , decompensated cirrhosis, hepatocellular carcinoma (HCC) (N = 4) , liver transplantation for HCC and septic shock with acute kidney failure
- None were related to study drugs
- Of the 5 patients with history of HCC, 2 had HCC recurrences during the treatment phase, 2 had de novo during study
- Hemoglobin < 10 g/dL, N = 4 (16%) ; < 8.5 g/ dL in 1 patient
Summary
- Retreating patients who failed a DAA-based regimen with NS5A/NS3 RASs with the combination of SOF + EBR/GZR + RBV for 16 weeks is efficacious and represent an interesting option
- Safety needs to be monitored cautiously in these patients with a severe disease