RUBY-II Study: ombitasvir/paritaprevir/ritonavir ± dasabuvir for HCV genotype 1a or 4 with severe renal impairment
Gane E. AASLD 2016, Abs. 935
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Genotype
1a
4
1a
4
Treatment history
Naive
Naive
Cirrhosis
No
No
Special population
Chronic Kidney disease
Chronic Kidney disease
Design
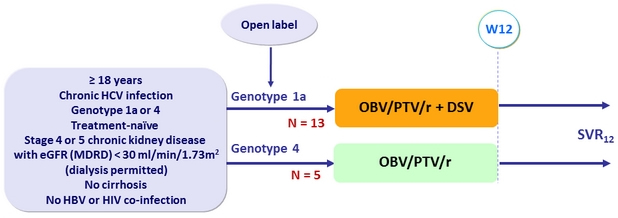
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg QD = 2 tablets
- Dasabuvir (DSV): 250 mg BID
Objective
- SVR12 (HCV RNA < 25 IU/mL), by ITT and mITT (exclusion of non-virologic failures)
Baseline characteristics and outcome
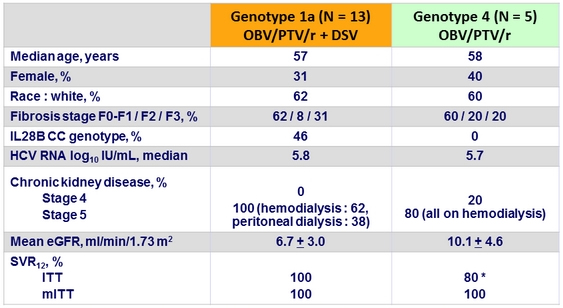
* The patient underwent elective renal transplantation and withdrew consent at treatment W2 and did not achieve SVR12
Adverse events and laboratory abnormalities, N (%)
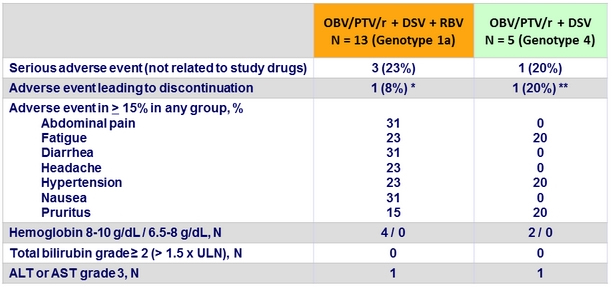
* Discontinued study drug (on treatment D77) due to grade 3 ALT, but achieved SVR12
** Discontinued study drug due to renal failure and transplantation at treatment W2, and did not achieve SVR12
Summary
- In this study of non-cirrhotic, treatment-naïve patients with stage 4 or 5 chronic kidney disease, including those receiving dialysis, the RBV-free regimen of OBV/PTV/r ± DSV resulted in ITT SVR12 rate of 100% for genotype 1a and 80% for genotype 4 (only 5 patients enrolled ), and a mITT SVR12 rate of 100% for genotypes 1a and 4, with no on-treatment virologic failure or relapse
- The RBV-free 2-DAA and 3-DAA regimens were generally well tolerated in this patient population
- Most adverse events were mild to moderate in severity, and there were no serious adverse event deemed related to study drugs
- These data suggest that RBV may not be necessary in some genotype 1a- or genotype 4-infected patients with severe renal impairment treated with OBV/PTV/r ± DSV
- larger trials are needed to confirm the results of this exploratory study