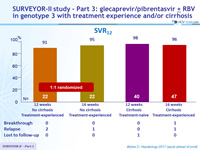
SURVEYOR-II study – Part 3: glecaprevir/pibrentasvir ± RBV in genotype 3 with treatment experience and/or cirrhosis
Wyles D. Hepatology 2017 ; Sept 19 (ePub)
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
3
3
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
Design
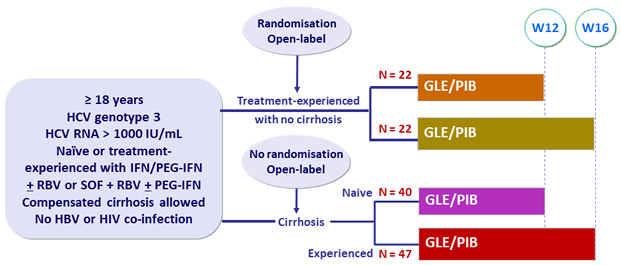
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA < 25 IU/ml), no formal statistical hypothesis
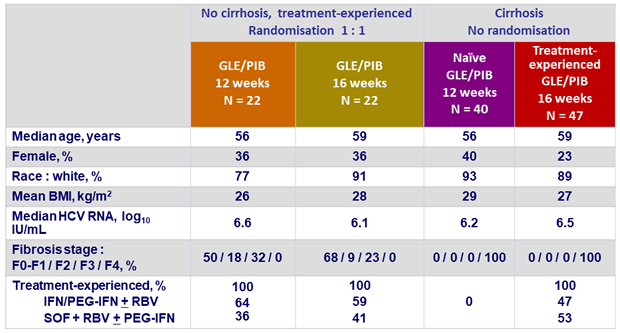
Baseline characteristics
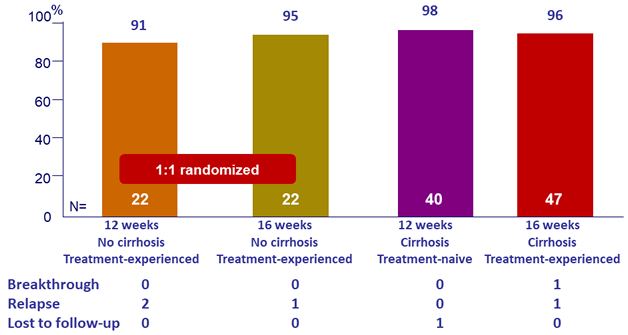
SVR12
Virologic failures
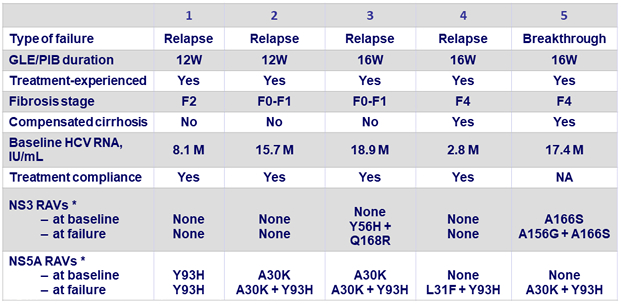
* RAVs detected by next-generation sequencing at 15% threshold :
? NS3 : 36, 43, 54, 55, 56, 80, 155, 156, 166, and 168
? NS5A : 24, 28, 29, 30, 31, 32, 58, 92, and 93
Adverse events and laboratory abnormalities
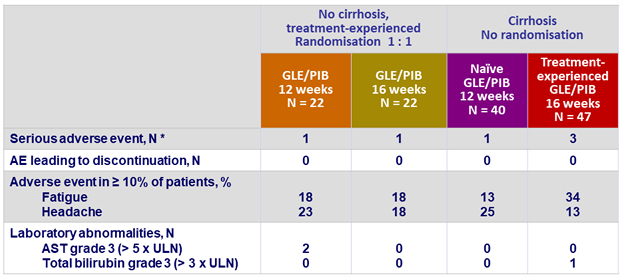
*
Umbilical hernia, colon cancer, pleural effusion, squamous cell carcinoma of the skin, schizophrenia, angina pectoris
Summary
- High efficacy with GLE/PIB in patients with HCV genotype 3 infection with compensated cirrhosis and/or prior treatment experience
- SVR12 rates of 98% and 96% in treatment-naïve and treatment-experienced patients with cirrhosis following 12 and 16 weeks of the QD combination, respectively
- SVR12 rate of 96% in treatment-experienced patients without cirrhosis following 16 weeks of treatment, or 91% after 12 weeks
- GLE/PIB was well tolerated
- with mostly mild adverse events
- no drug related-related serious adverse event
- few occurrences of grade 3 or higher laboratory abnormalities
- and no study drug discontinuation