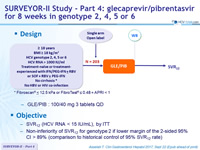
SURVEYOR-II Study - Part 4: glecaprevir/pibrentasvir for 8 weeks in genotype 2, 4, 5 or 6
Asselah T. Clin Gastroenterol Hepatol 2017 ; Sept 22 (Epub)
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
2
4
6
2
4
6
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
No
No
Design
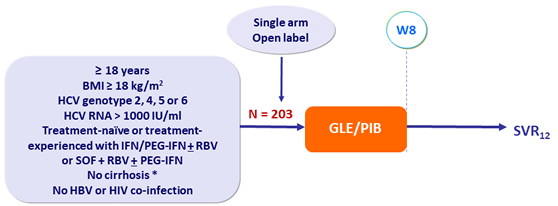
* Fibroscan® ≤ 12.5 kPa or FibroTest® ≤ 0.48 + APRI < 1
- GLE/PIB : 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA< 15 IU/mL), by ITT
- Non-inferiority of SVR12 for genotype 2 if lower margin of the 2-sided 95% CI > 89% (comparison to historical control of 95% SVR 12 rate)
Baseline characteristics and SVR12
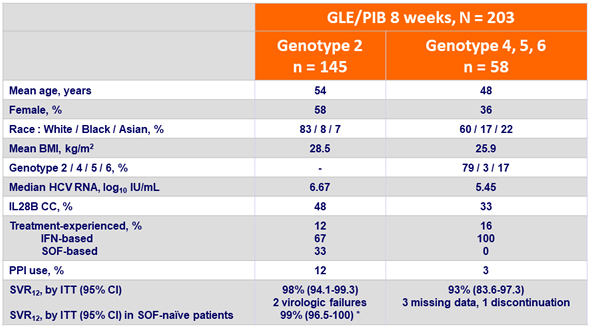
* Non- inferior to historical SVR 12 of 95%
Adverse events and laboratory abnormalities, %
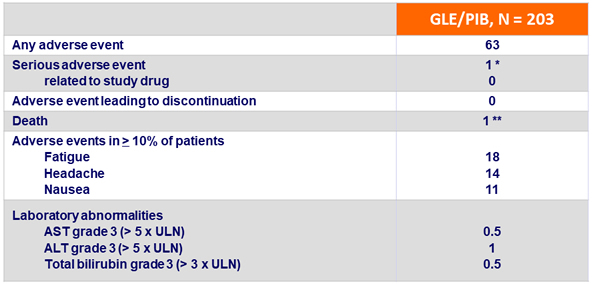
*
Cholecystitis (Day 30 of treatment) in 1 patient, urosepsis (post-treatment Day 15) in 1 patient, both assessed as not related to study drug
Summary
- 97% of genotype 2, 4, 5, or 6-infected patients without cirrhosis achieved SVR12 following 8 weeks of GLE/PIB
- In DAA-naïve patients with genotype 2, 8-weeks of GLE/PIB yielded a SVR12 rate of 99%, that was non-inferior to the historical 95% SVR12 rate achieved with 12 weeks of SOF + RBV
- There were no virologic failures in patients with genotype 4, 5 or 6 infection
- SVR12 was not impacted by baseline HCV RNA, genotype/subtype, F0-F3 fibrosis stage, baseline polymorphisms, and prior treatment experience with IFN/PEG-IFN- or SOF-based regime ns SVR rates were similar to those following 12-weeks with GLE/PIB
- In patients with genotype 2 infection, 42% (53/126) had NS5A RAS 31M at baseline ; 96% (51/53) of these patients achieved SVR 12
- The 2 relapses occurred in treatment-experienced patients with genotype 2a and baseline NS5A RAS L31M, with no treatment-emergent RAS at failure
- GLE/PIB for 8 weeks was well-tolerated, with no discontinuations due to adverse event, no drug-related serious adverse event, and rare grade 3 or higher laboratory abnormalities