QUARTZ II-III : OBV/PTV/r + SOF RBV in genotype 2 or 3
Shafran SD. J. Viral Hep 2018; 25:118-25.
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Sofosbuvir
Paritaprevir/ritonavir
Ombitasvir
Sofosbuvir
Genotype
2
3
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
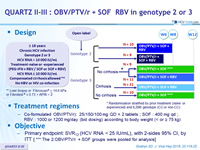
Design
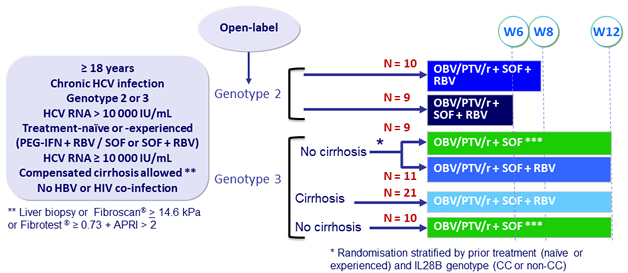
Treatment regimens
-
Co-formulated OBV/PTV/r ): 25/150/100 mg QD = 2 tablets ; SOF : 400 mg qd ; RBV : 1000 or 1200 mg/day (bid dosing) according to body weight (< or ≥ 75 kg )
Objective
- Primary endpoint: SVR12 (HCV RNA < 25 IU/mL), with 2-sides 95% CI, by ITT [ *** The 2 OBV/PTV/r + SOF groups were pooled for analysis]
Baseline characteristics and SVR12
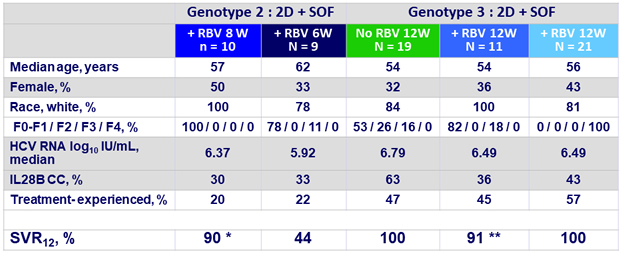
* 1 relapse (no treatment-emergent substitutions), this patient had previously failed SOF + RBV
** 1 discontinuation of treatment on D8 due to an adverse event of influenza-like illness deemed unrelated to study drugs ; this patient did not achieve SVR12
Adverse events and laboratory abnormalities
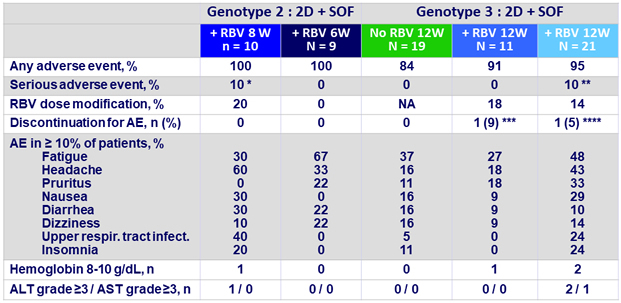
* Pneumonia on D26 unrelated to DAA ;
** 1 respir . tract infection on D33 ; 1 anemia on D28 , both unrelated to DAA;
***on D8 due to influenza - like symptoms unrelated to DAA ;
****on D78 due vertigo possibly related to DAA
Summary
- In patients with genotype 3 and no cirrhosis, high SVR rates were seen with 2D + SOF with or without RBV for 12 weeks
- 100% without RBV and 91% with RBV (1 discontinuation for AE)
- In patients with genotype 3 and compensated cirrhosis, SVR12 was 100% after 12 weeks of 2D + SOF + RBV
- In patients with genotype 2 and no cirrhosis, an 8-week regimen of 2D + SOF + RBV achieved an SVR12 rate of 90 %
- Shortening treatment duration to 6 weeks resulted in a high rate of relapse
- 2D + SOF ± RBV had a favorable safety profile consistent with previous studies
- Conclusion : 2D + SOF ± RBV may be a useful treatment option for patients with genotype 3 infection with or without cirrhosis
- Limitation : small sample size