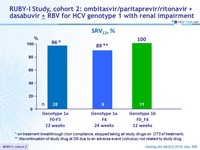
RUBY-I Study, cohort 2: ombitasvir/paritaprevir/ritonavir + dasabuvir ± RBV for HCV genotype 1 with renal impairment
Vierling JM. AASLD 2016, Abs. 886
Anti-HCV
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Paritaprevir/ritonavir
Ombitasvir
Dasabuvir
Ribavirin
Genotype
1a
1b
1a
1b
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
Yes
No
Yes
No
Special population
Chronic Kidney disease
Chronic Kidney disease
Design
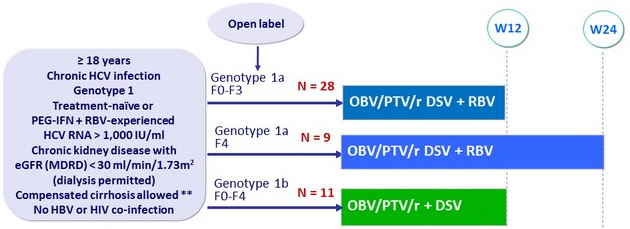
** Liver biopsy ( Metavir = F3, Ishak score = 4) or F ibroscan < 12.5 kPa or FibroTest = 0.72 + APRI = 2
Treatment regimens
- Co-formulated ombitasvir (OBV)/ paritaprevir (PTV)/ rironavir (r): 25/150/100 mg qd = 2 tablets
- Dasabuvir (DSV): 250 mg bid ; RBV 200 mg qd (genotype 1a)
Objective
- SVR12, (HCV RNA < 25 IU/ml) by intent-to-treat with 2-sided 95% CI
Baseline characteristics
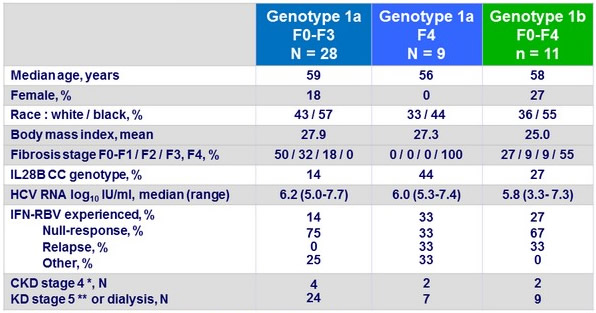
* eGFR 15- 30 ml/min/1.73 m² ; ** eGFR < 15 ml/min/1.73 m²
SRV12, %
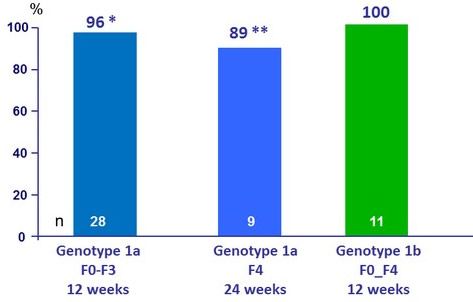
* on - treatment breakthrough (non compliance , stopped taking all study drugs on D73 of treatment)
** discontinuation of study drug at D6 due to an adverse event (volvulus) not related to study drug
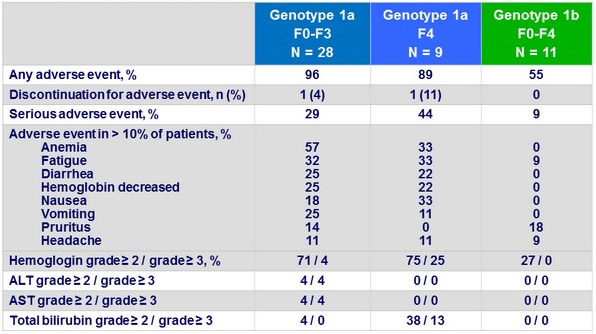
Adverse events and laboratory abnormalities
Summary
- OBV/PTV/r + DSV ± RBV resulted in an SVR12 rate of 96 % in patients with CKD stages 4 or 5 in cohort 2 of the RUBY-I study
- The regimen was generally well tolerated for this group of patients with severe underlying comorbidities, with
- 1 possibly DAA-related serious adverse event of diarrhea
- and 1 discontinuation due to an adverse event unrelated to treatment
- A large proportion of patients on RBV required RBV dose modification for anemia
- Most adverse events were mild or moderate in severity
- These results of efficacy and safety support the use of this regimen in patients with advanced renal disease, for whom treatment options are limited