SURVEYOR-I et II study – Part 2 in cirrhosis: Glecaprevir + Pibrentasvir in genotypes 1 or 3
Gane E. Gastroenterology 2016;151:651-9
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
3
1
3
Treatment history
Naive
Naive
Cirrhosis
Yes
Yes
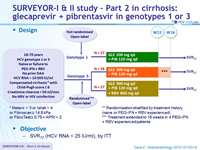
Design
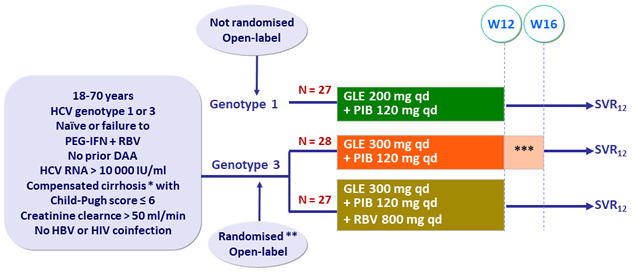
* Metavir > 3 or Ishak > 4 or Fibroscan ≥ 14.6 kPa or FibroTest ≥ 0.75 + APRI > 2
** Randomisation stratified by treatment history (naive or PEG-IFN + RBV experienced)
*** Treatment extended to 16 weeks in 4 PEG-IFN
+ RBV experienced patients
Objective
- SVR12 (HCV RNA< 25 IU /ml), by ITT
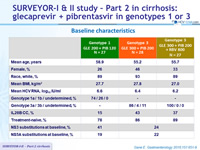
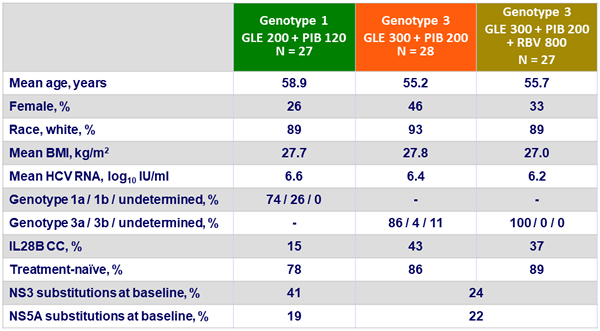
Baseline characteristics
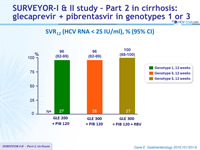
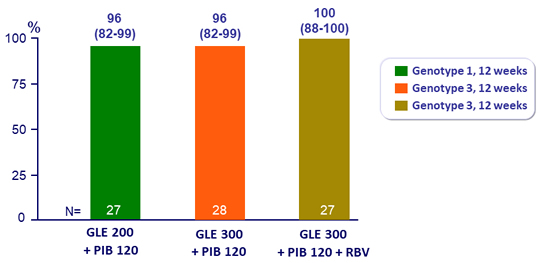
SVR12 (HCV RNA < 25 IU/ml), % (95% CI)
Relapses
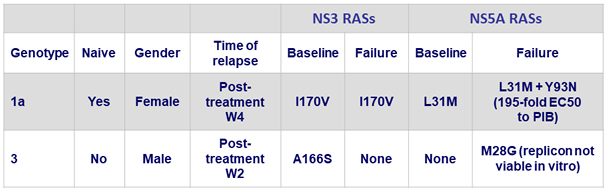
Resistance analysis (population sequencing with 15% threshold)
- SVR12 was achieved in 100% of patients without baseline substitutions and efficacy remained high in the presence of baseline substitutions
- All 11 genotype 3-infected patients with baseline NS5A substitutions, including 4 patients with additional NS3 substitutions, achieved SVR12
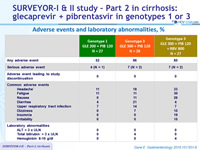
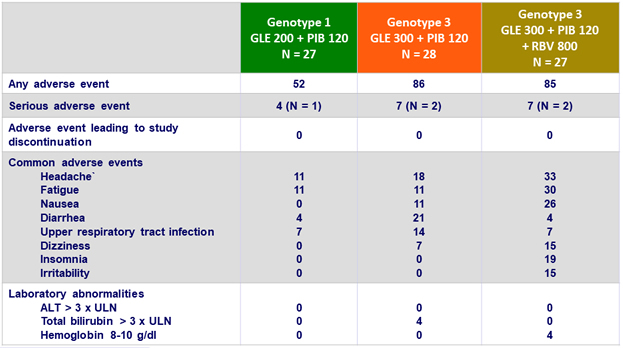
Adverse events and laboratory abnormalities, %
Summary
- In this phase 2 study, the once-daily combination of Glecaprevir and Pibrentasvir for 12 weeks is sufficient to achieve high rates of SVR12 in patients with genotype 1 or genotype 3 infection and compensated cirrhosis
- Adverse events were mostly mild in severity
- Potential for a pangenotypic therapy without RBV co-administration