HEPNET ACUTE HCV IV study: 6 weeks LDV/SOF for acute hepatitis C
Deterding K, Lancet Infect Dis 2017; 17:215-22
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1a
1b
1a
1b
Special population
Acute HCV infection
Acute HCV infection
Design
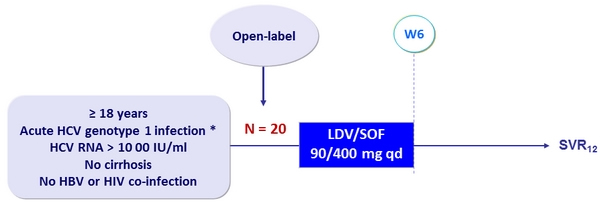
* Documented seroconversion to HCV antibody positivity within the 4 months before screening, or known or suspected exposure to HCV within the 4 months before screening with ALT > 10 x ULN at screening or within a 4-week period before screening
Objective
- SVR12 (HCV RNA < 15 UI/ ml), power of 80% to conclude efficacy if the true response rate is > 98% (lower margin of the 95 % CI > 80%)
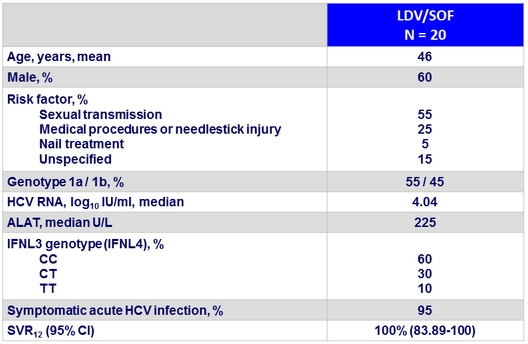
Baseline characteristics and outcome
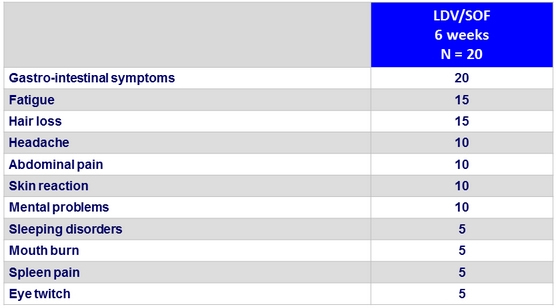
Adverse events, %
Summary
- A short course of 6 weeks with an interferon-free and ribavirin-free therapy consisting of LDV/SOF fixed drug combination resulted in a SVR12 of 100% in patients with acute HCV genotype 1
- Rapid improvement of symptoms and biochemical abnormalities of acute hepatitis
- Very good tolerance




