AL-335-604 study: AL-335 + ODV ± SMV in naïve patients, phase II
Gane E. AASLD 2016, Abs. PS-153
Anti-HCV
Simeprevir
Odalasvir
AL-335
Simeprevir
Odalasvir
AL-335
Genotype
1
3
1
3
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
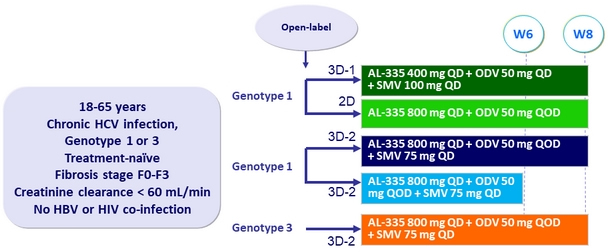
- AL-335: 400 or 800 mg QD
- ODV: 25 mg QD, 50 mg QD or every other day (QOD)
- SMV: 75 or 100 mg QD
Objective
- Primary endpoint: SVR12 (HCV RNA< LLOQ)
Baseline characteristics and outcome
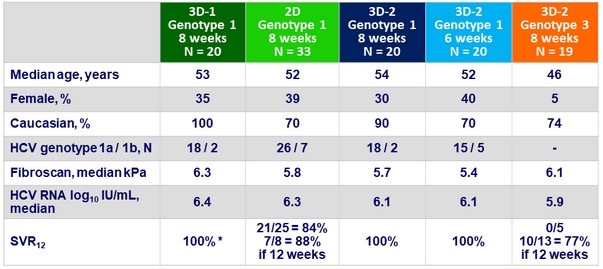
* 2 premature discontinuations: 1 due to an AE, 1 due to entry criterion violation
Adverse events, N
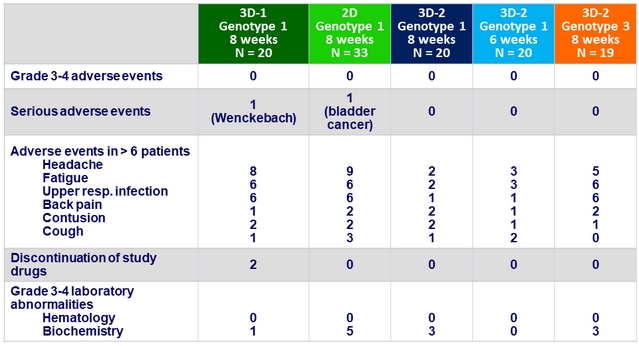
Summary
- HCV genotype 1, treatment naïve patients without cirrhosis achieved SVR12 of 100% with AL-335 + ODV + SMV for 6 or 8 weeks
- Tolerability was good
- Further development in phase 3
- Inadequate efficacy
- In genotype 1 of AL-335 + ODV, without SMV
- In genotype 3 of AL-335 + ODV + SMV
- Development halted
- Phase IIb and III studies with AL-335 800 mg + ODV 25 mg + SMV 75 mg QD




