C-WORTHY Study part D: grazoprevir + elbasvir + RBV in genotype 3
Efficacy of 12 or 18 Weeks of Grazoprevir plus Elbasvir with Ribavirin in Treatment-Naive, Noncirrhotic HCV Genotype 3–Infected Patients
Gane E. J Viral Hepat. 2017 ; 10 :895-9
Anti-HCV
Grazoprevir
Elbasvir
Ribavirin
Grazoprevir
Elbasvir
Ribavirin
Genotype
3
3
Treatment history
Naive
Naive
Cirrhosis
No
No
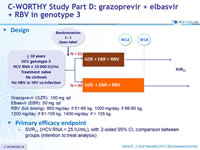
Design
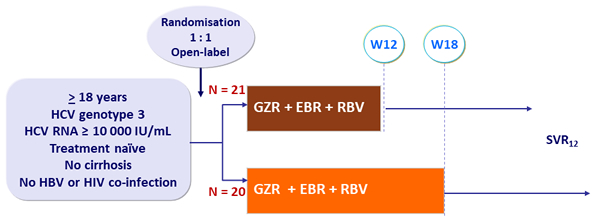
- Grazoprevir (GZR): 100 mg qd
- Elbasvir (EBR): 50 mg qd
- RBV (bid dosing): 800 mg/day if 51-65 kg, 1000 mg/day if 66-80 kg, 1200 mg/day if 81-105 kg, 1400 mg/day if > 105 kg
Primary efficacy endpoint
- SVR12 (HCV RNA < 25 IU/ml), with 2-sided 95% CI, comparison between groups (intention to treat analysis)
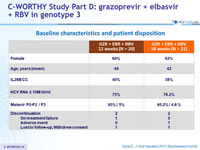
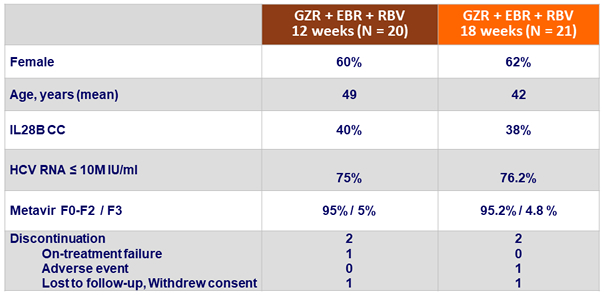
Baseline characteristics and patient disposition
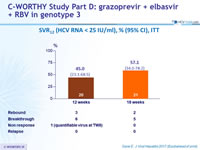
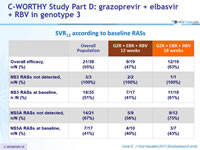
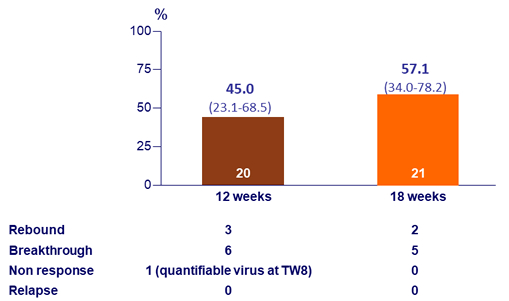
SVR12 (HCV RNA < 25 IU/ml), % (95% CI), ITT
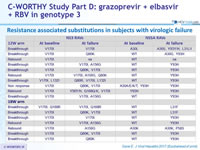
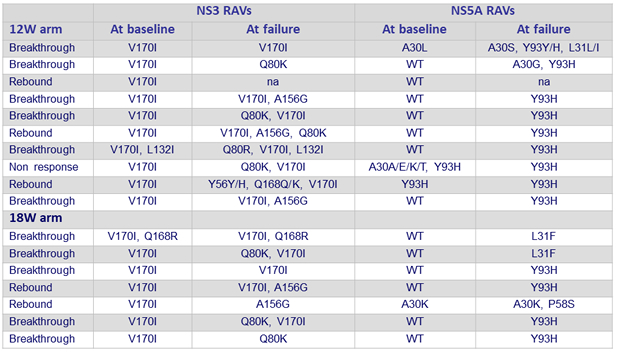
Resistance associated substitutions in subjects with virologic failure
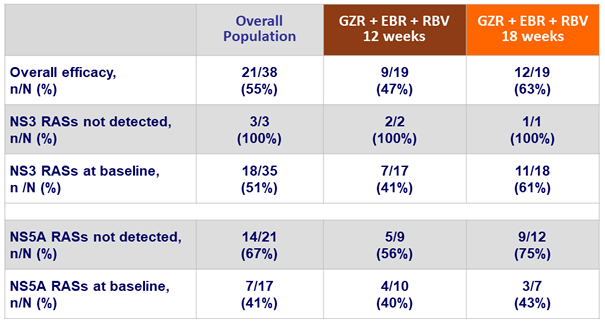
SVR12 according to baseline RAVs
Adverse events, N (%)
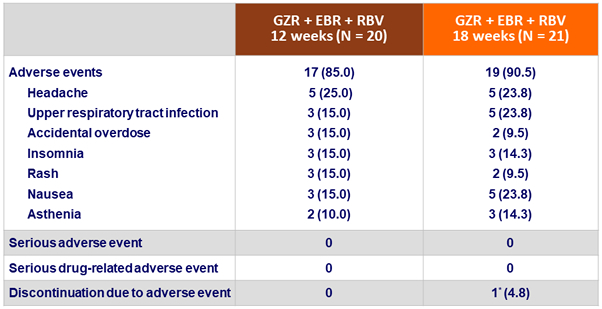
* dyspnea , fatigue and asthenia 2-3 days after starting treatment
Summary
- Efficacy of 12 or 18 weeks of GZR + EBR + RBV in HCV genotype 3 infected patients
- Was suboptimal due to on-treatment virologic failure in 17 of 41 patients
- No subject relapsed after the end of therapy
- NS5A RASs found in 16 of 17 failures, Y93H the most common NS5A RAS identified
- GZR + EBR + RBV was generally safe and well tolerated
- Adverse events were slightly more common in the 18-week arm compared with the 12-week arm
- No differences in serious adverse events or laboratory abnormalities
- Adverse event considered to be severe in intensity reported in only 1 patient (non-drug related depression during follow-up)