MAGELLAN-I Study, Part 2 : Glecaprevir, pibrentasvir, genotype 1, genotype 4, NS3-experienced, NS5A-experienced, cirrhosis, no cirrhosis
Poordad F, Hepatology. 2018;671253-1260 & Pilot-Matias T, EASL 2017, Abs. SAT-204
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
4
1
4
Treatment history
PI (NS3)-experienced
NS5A experienced
PI (NS3)-experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
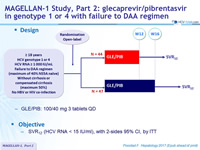
Design
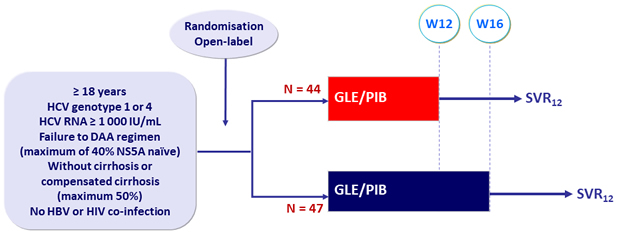
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
- SVR12 (HCV RNA< 25 IU /ml), with 2-sides 95% CI, by ITT
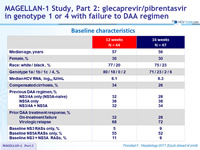
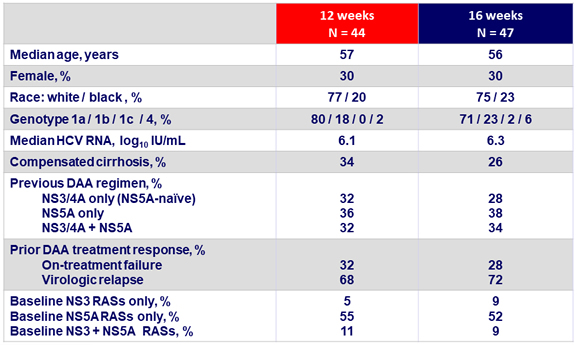
Baseline characteristics
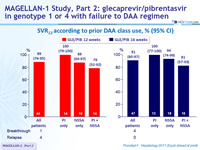
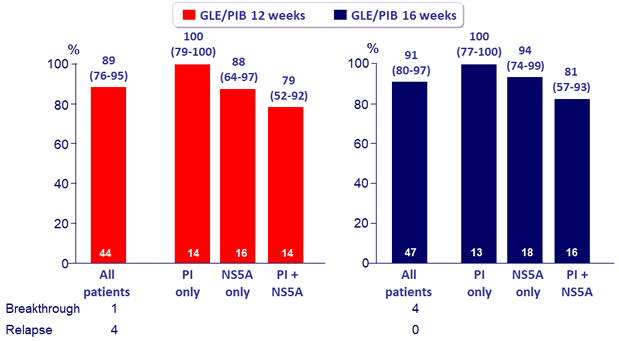
SVR12 according to prior DAA class use, % (95% CI)
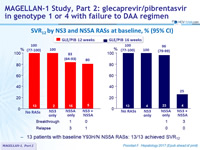
SVR12 by NS3 and NS5A RASs at baseline, % (95% CI)
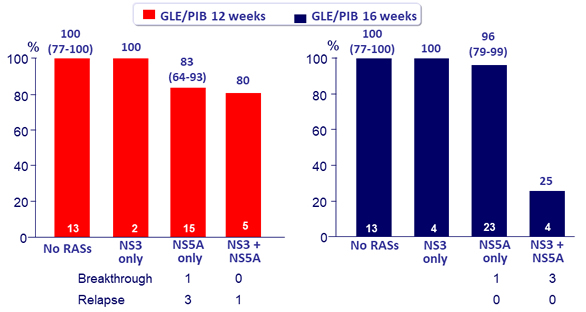
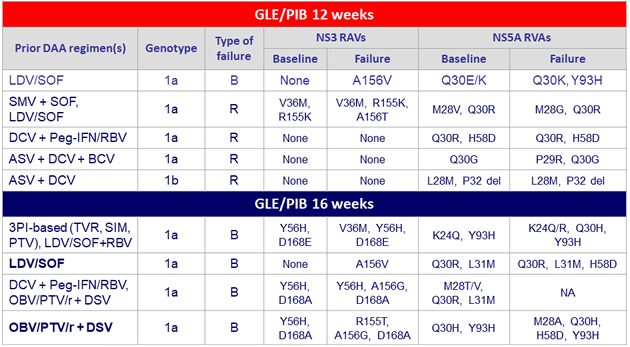
- 13 patients with baseline Y93H/N NS5A RASs : 13/13 achieved SVR12
9 virologic failures : 4 relapses (R) and 5 virologic breakthroughs (B)
Adverse events and laboratory abnormalities, %
Summary
- In patients with prior PI failure and NS5A inhibitor-naïve, SVR12 of 100% after 12 or 16 weeks of glecaprevir / pibrentasvir
- In patients with prior failure to both PI and NS5A inhibitor, lower SVR12 were achieved
- In patients with with prior failure to NS5A inhibitor, SVR12 was 94% with 16 weeks of GLE/PIB, with no relapse
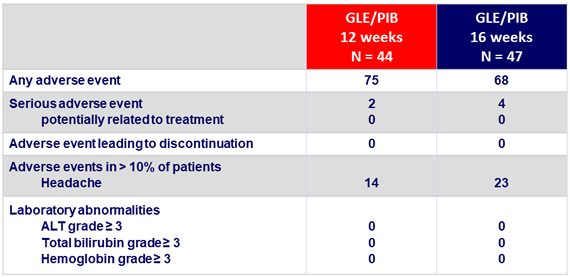
- Good tolerability, with no treatment-related serious adverse event –
- No grade 3 or 4 laboratory abnormalities
- No discontinuation due to adverse events