EXPEDITION-I : glecaprevir, pibrentasvir, genotype1, genotype 2, genotype 4, naïve, IFN-experienced, SOF-experienced, cirrhosis
Forns X. Lancet Infect Dis 2017; 17:1062-8
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
2
4
1
2
4
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
Yes
Yes
Design
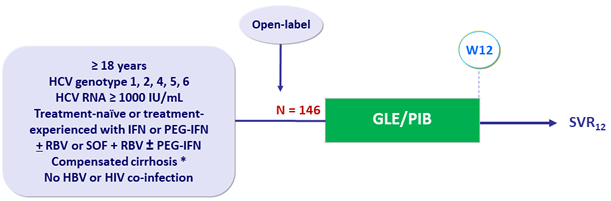
* Liver biopsy (Metavir 4 or Ishak = 4, FibroTest = 0.75 + APRI = 2 or FibroScan = 14.6 kPa ; Child- Pugh score = 6
- GLE/PIB: 100/40 mg 3 tablets QD
Objective
-
Primary endpoint: SVR12 (HCV < 15 IU/mL), with 2–sided 95% CI, by ITT
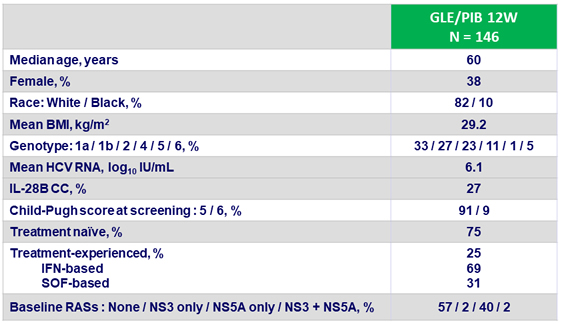
Baseline characteristics
SVR12 (HCV RNA < 15 IU/mL), by ITT, % (95% CI)
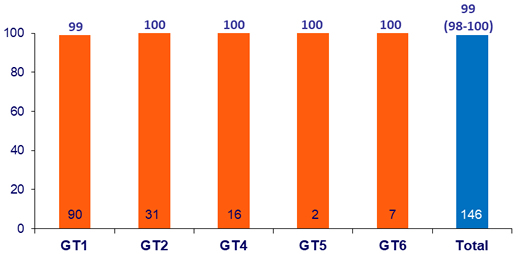
- One patient with genotype 1a
and history of non- reponse to Peg-IFN + RBV relapsed at post-treatment week 8
- NS5A RASs: Y93N at baseline, Y93N, Q30R and H58D present at failure
- NS3 RASs: none
Adverse events and laboratory abnormalities, %
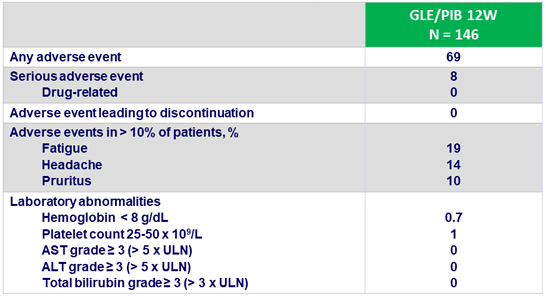
- Occurrence of hepatocellular carcinoma in 2 patients
- One patient with a history of hemophilia died 61 days after completing treatment (cerebral hemorrhage, not related to study drugs)
Summary
- Glecaprevir / pibrentasvir for 12 weeks achieved a 99% SVR12 rate in patients with compensated cirrhosis and genotype 1, 2, 4, 5 or 6
- Treatment was well- tolerated
- no elevations in alanine aminotransferase
- and no treatment discontinuations due to adverse events





