MAGELLAN-2 Study: glecaprevir/pibrentasvir in liver or kidney transplanted patients
Reau N. Hepatology 2018;68:1298-1307
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
2
3
1
2
3
Treatment history
Naive
IFN-Experienced
Naive
IFN-Experienced
Cirrhosis
No
No
Special population
Liver transplantation
Kidney transplantation
Liver transplantation
Kidney transplantation
Design
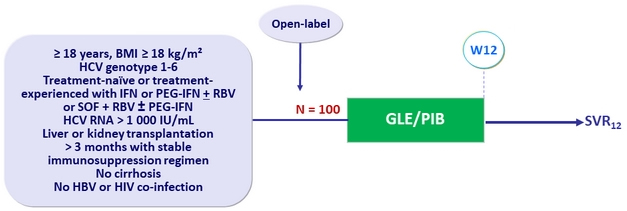
- GLE/PIB: 100/40 mg 3 tablets QD
- Patients enrolled in Australia, Canada, Italy, New Zealand, Puerto Rico, Spain, Taiwan, the United Kingdom and the United States
Objective
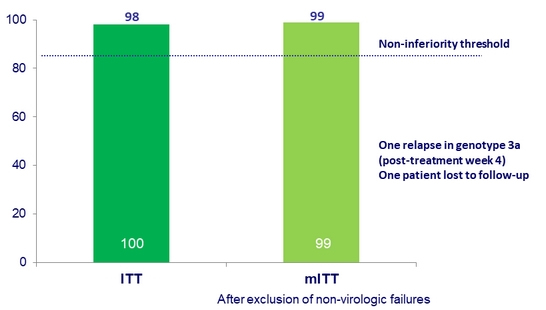
- Primary endpoint: SVR12 ( HCV < 15 IU/mL)
- Non-inferiority to historical 94% SVR 12 standard-of-care rate, achieved if > 86% (8% margin)
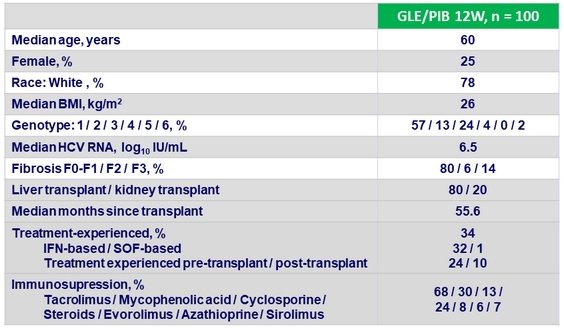
Baseline characteristics
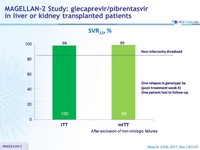
SVR12, %
Adverse events and laboratory abnormalities, N (%)
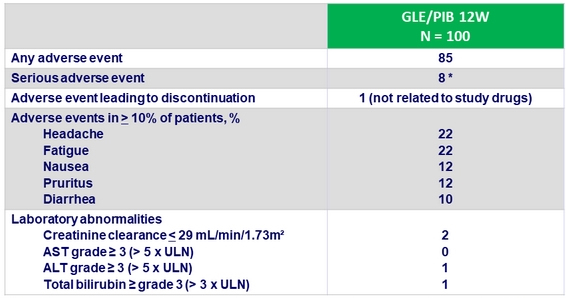
* 2 related to study drugs : sinusitis (Day 2), abnormal hepatic function (post-treatment week 4)
- One patient experienced mild liver transplant rejection, unrelated to study drugs
Summary
- Glecaprevir/pibrentasvir for 12 weeks achieved a 99% SVR12 rate in patients with liver or kidney transplant and genotype 1-6
- This rate was not inferior to historical standard of care
- Treatment was well-tolerated