ENDURANCE-3 Study: glecaprevir/pibrentasvir vs SOF + DCV in genotype 3 without cirrhosis
Zeuzem S. NEJM 2018;378:354-69
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
3
3
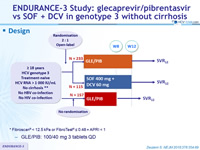
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
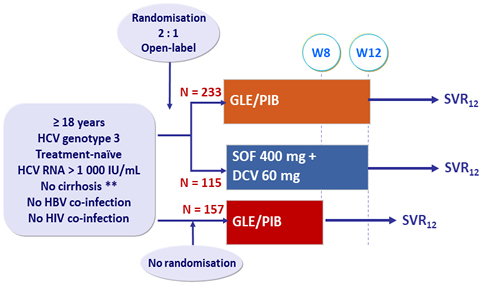
* Fibroscan® < 12.5 kPa or FibroTest® = 0.48 + APRI < 1
- GLE/PIB: 100/40 mg 3 tablets QD
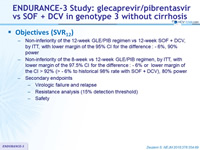
Objectives (SVR12)
- Non-inferiority of the 12-week GLE/PIB regimen vs 12-week SOF + DCV , by ITT, with lower margin of the 95% CI for the difference : - 6 %, 90% power
- Non-inferiority of the 8-week vs 12-week GLE/PIB regimen, by ITT, with lower margin of the 97.5% CI for the difference : - 6% or lower margi n of the CI > 92% (> - 6% to historical 98% rate with SOF + DCV), 80% power
- Secondary endpoints
- Virologic failure and relapse
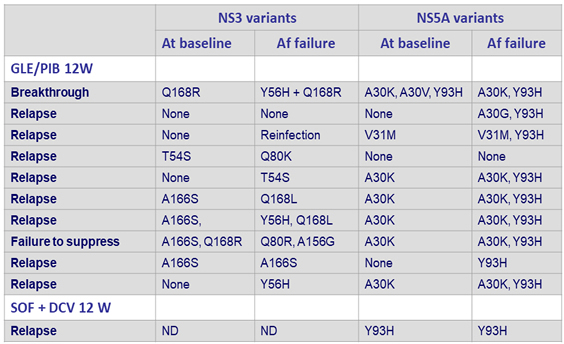
- Resistance analysis (15% detection threshold)
- Safety
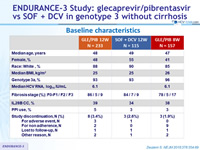
Baseline characteristics
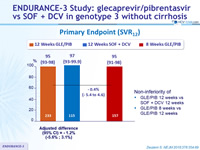
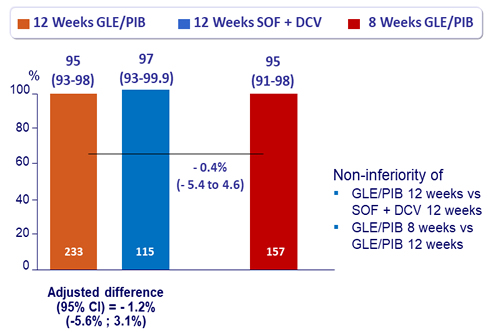
Primary Endpoint (SVR12)
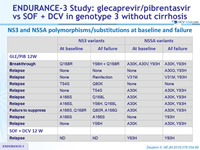
NS3 and NS5A polymorphisms/substitutions at baseline and failure
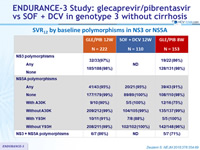
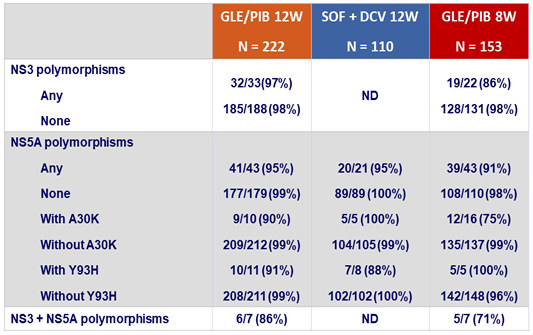
SVR12 by baseline polymorphisms in NS3 or NS5A
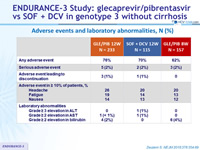
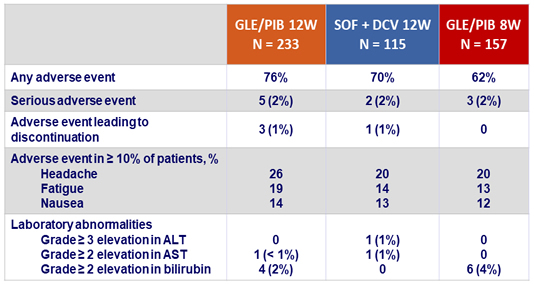
Adverse events and laboratory abnormalities, N (%)
Summary
- Once-daily treatment with GLE/PIB for either 8 weeks or 12 weeks achieved high rates of sustained virologic response among patients with HCV genotype 3 infection who did not have cirrhosis
- 95% of patients achieved SVR 12 with 8 weeks of GLE/PIB
- GLE/PIB was well tolerated
- No patient discontinued for adverse event in GLE/PIB 8 weeks group
- Only 3 patients (1%) discontinued study drugs for adverse event in GLE/PIB 12 weeks group
- No significant laboratory abnormalities