STREAGER Study: EBR/GZR for 8 weeks in genotype 1b without severe fibrosis
Abergel A. EASL 2018, Abs. LBP-010
Anti-HCV
Grazoprevir
Elbasvir
Grazoprevir
Elbasvir
Genotype
1b
1b
Treatment history
Naive
Naive
Cirrhosis
No
No
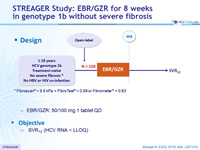
Design
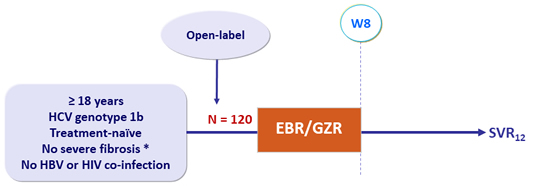
*
Fibroscan ® < 9.5 kPa + FibroTest ® < 0.59 or Fibrometer ® < 0.63
- EBR/GZR: 50/100 mg 1 tablet QD
Objective
- SVR12, (HCV RNA < LLOQ)
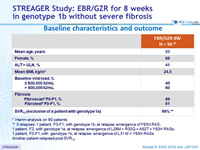
Baseline characteristics and outcome
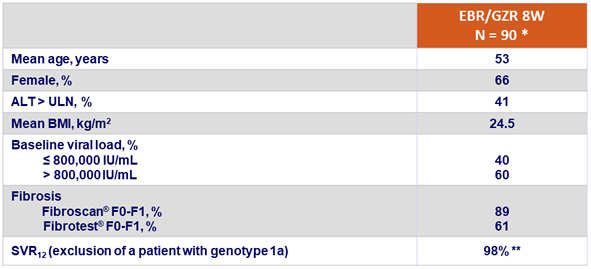
* Interim analysis on 90 patients
** 3 relapses: 1 patient, F0-F1, with genotype 1b, at relapse: emergence of Y93H RAS ; 1 patient, F2, with genotype 1e, at relapse: emergence of L28M + R30Q + A92T + Y93H RASs, 1 patient, F0-F1, with genotype 1b, at relapse: emergence of L31 M + Y93H RASs
Another patient relapsed post SVR12
Safety
- No adverse event of grade 3 or 4
- Adverse events >10% related to treatment :
- Asthenia : 28%
- Headache : 23%
- Digestive disorders : 13%
Summary
- High cure rate (SVR12 98%) was achieved in a treatment-naïve non severe fibrosis GT1b-infected population treated for 8 weeks with the combination of EBR/GZR
- Good safety profile
- These results are
- in agreement with the results obtained in the C-WORTHY study with 8 weeks of EBR/GZR ± RBV (SVR12 of 97%)
- very similar to those obtained in patients treated 12 weeks with EBR/GZR (SVR12 of 98%)
- These results are preliminary