LDV/SOF for 12 weeks in patients with genotype 1 and severe renal impairment
Lawitz E. AASLD 2017, Abs. 1587
Anti-HCV
Ledipasvir
Sofosbuvir
Ledipasvir
Sofosbuvir
Genotype
1
1
Treatment history
IFN-Experienced
IFN-Experienced
Design
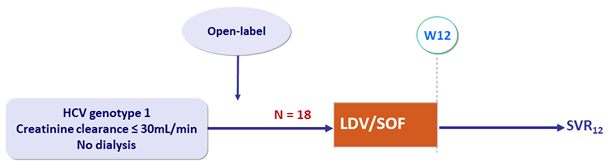
- LDV/SOF : 90/400 mg 1 tablet QD
Objective
- SVR12 (HCV RNA < 15 IU/ml)
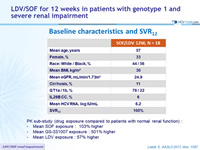
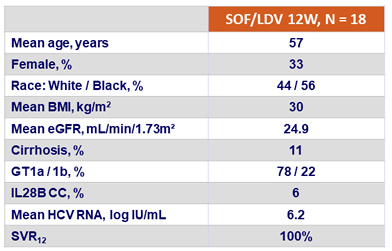
Baseline characteristics and SVR12
PK sub-study ( drug exposure compared to patients with normal renal function ) :
- Mean SOF exposure : 103% higher
- Mean GS-331007 exposure : 501% higher
- Mean LDV exposure : 57% higher
Adverse events, %
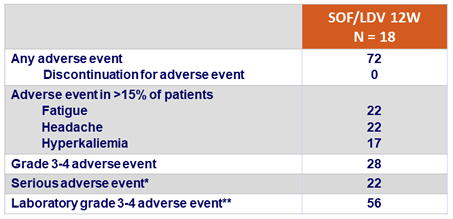
*Acute kidney injury and noncardiac chest pain (n=1), ; dehydration and hypotension (n=1); acute renal failure (n=1); hypotension and syncope (n=1)
**Elevated creatinine (Grade 3, n=3; Grade 4, n=1); decreased hemoglobin (Grade 3, n=3; Grade 4, n=1); elevated glucose (Grade 3, n=3); decreased bicarbonate (Grade 3, n=1); decreased lymphocytes (Grade 3, n=3)
Summary
- 12 weeks of LDV/SOF led to a 100% SVR 12 rate in patients with genotype 1 infection and severe renal impairment not undergoing dialysis
- Treatment was safe and well tolerated
- Plasma concentrations of the SOF metabolite GS-3310007 were close to 6-fold higher than in the LDV/SOF phase 2-3 trials