C-BREEZE-2 Study: ruzasvir + uprifosbuvir for 12 weeks in genotype 1-6
Lawitz E. AASLD 2017, Abs. 61
Anti-HCV
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Ruzasvir (MK-8408)
Uprifosbuvir (MK-3682)
Genotype
1a
1b
2
3
4
6
1a
1b
2
3
4
6
Treatment history
Naive
Naive
Cirrhosis
No
No
Design
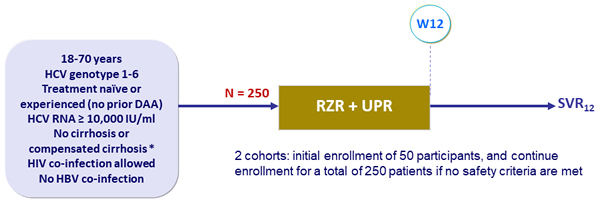
* Liver biopsy or Fibroscan ® > 12.5 kPa or FibroSure ® > 0.75 + APRI > 2
- RZR : 180 mg QD ; UPR : 450 mg QD
Objective
- SVR12 (HCV RNA < 15 IU/mL), full analysis set (FAS) : all participants who received = 1 dose of study medication ; mFAS : exclusion of participants with non- virologic failure
Baseline characteristics and SVR12
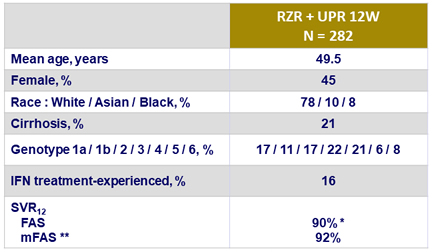
* 19 relapses , 2 discontinuations due to drug-related adverse events
** 8 patients excluded (7 discontinuations, 1 lost to follow -up)
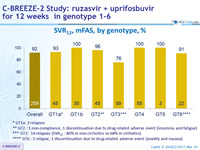
SRV12, mFAS , by genotype, %
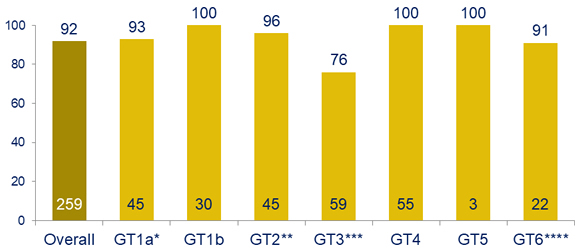
* GT1a: 3 relapses
** GT2 : 1 non-compliance, 1 discontinuation due to drug-related adverse event (insomnia and fatigue)
*** GT3: 14 relapses (SVR12 : 80% in non-cirrhotics vs 68% in cirrhotics)
**** GT6 : 1 relapse, 1 discontinuation due to drug-related adverse event (anxiety and nausea)
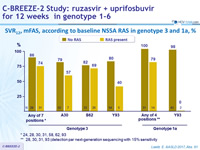
SVR12, mFAS, according to baseline NS5A RAS in genotype 3 and 1a, %
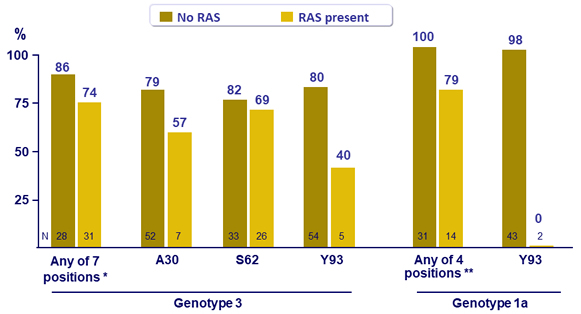
* 24, 28, 30, 31, 58, 62, 93
** 28, 30, 31, 93 (detection par next-generation sequencing with 15% sensitivity)
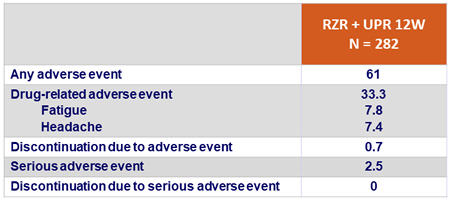
Adverse events, %
Summary
- Overall efficacy of the 2-drug regimen of ruzasvir + uprifosbuvir is suboptimal
- Lower efficacy in genotype 3; baseline RAS account for many failures
- High efficacy in other genotypes; potential impact of baseline Y93 RAS in GT1a, no impact of baseline RAS in other non-GT3 genotypes
- Good safety profile