MAGELLAN-3 Study: GLE/PIB + SOF + RBV in patients who failed GLE/PIB
Wyles D. J Hepatol. 2019 Mar 8 (Epub ahead of print)
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Sofosbuvir
Ribavirin
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Sofosbuvir
Ribavirin
Genotype
1
1a
3
1
1a
3
Treatment history
PI (NS3)-experienced
NS5A experienced
PI (NS3)-experienced
NS5A experienced
Cirrhosis
Yes
No
Yes
No
Design
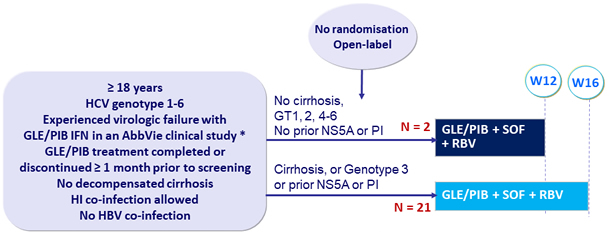
* SURVEYOR-2, ENDURANCE-3, MAGELLAN-1, EXPEDITION-1
- GLE/PIB: 100/40 mg 3 tablets QD
- SOF: 400 mg QD
- RBV: 1000-1200 mg (BID regimen)
Primary endpoint
- SVR12 (HCV < LLOQ)
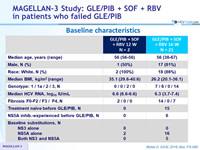
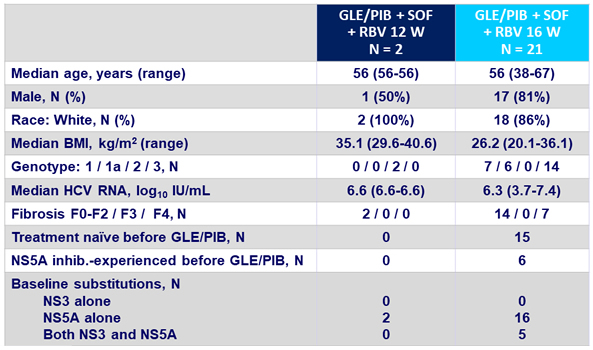
Baseline characteristics
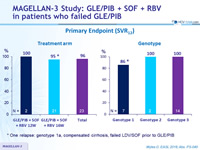
Primary Endpoint (SVR12)
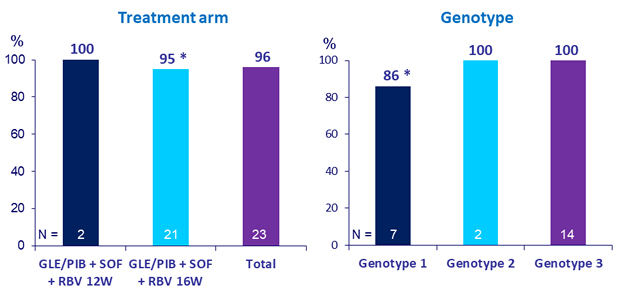
* One relapse: genotype 1a, compensated cirrhosis, failed LDV/SOF prior to GLE/PIB
Adverse events and laboratory abnormalities, N (%)
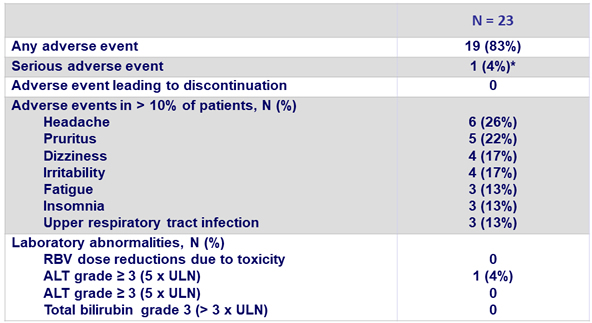
*Not related to study drugs, not leading to drug discontinuation
Summary
- GLE/PIB + SOF + RBV resulted in 96% SVR12 in patients with previous GLE/PIB virologic failure
- 100% SVR12 in the 14 patients with genotype 3
- Treatment was well-tolerated