EXPEDITION-V Study: GLE/PIB in patients with renal impairment
Gane E. N Engl J Med. 2017;377:1448-55
Anti-HCV
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Glecaprevir (ABT-493)
Pibrentasvir (ABT-530)
Genotype
1
2
3
1
2
3
Treatment history
Naive
IFN-Experienced
SOF-experienced
Naive
IFN-Experienced
SOF-experienced
Cirrhosis
Yes
No
Yes
No
Special population
Chronic Kidney disease
Chronic Kidney disease
Design
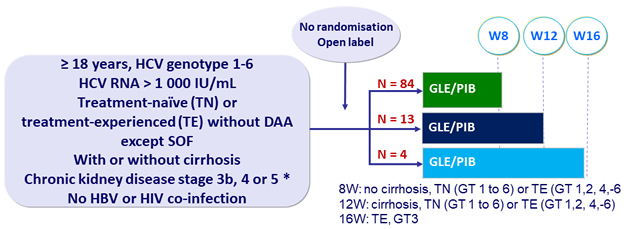
* stage 3b: eGFR = 30 to < 45 mL/min/1.73m² ;
stage 4: eGFR = 15 to < 30 mL ; stage 5: eGFR<15 mL or dialysis-dependent
- GLE/PIB: 100/40 mg 3 tablets QD, administered regardless of timing of dialysis (hemo- or peritoneal)
Primary endpoint
- SVR12 (HCV < LLOQ)
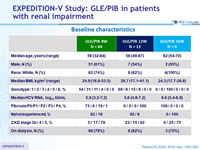
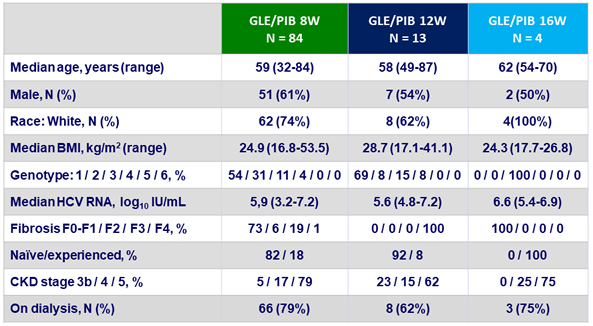
Baseline characteristics
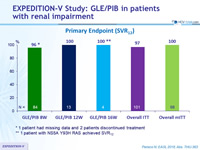
Primary Endpoint (SVR12)
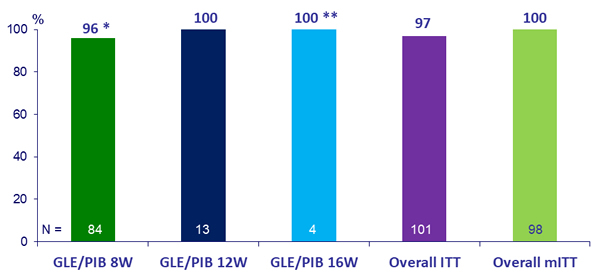
* 1 patient had missing data and 2 patients discontinued treatment
** 1 patient with NS5A Y93H RAS achieved SVR12
Adverse events and laboratory abnormalities, %
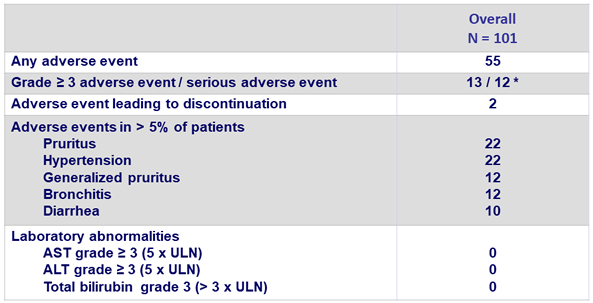
* No AE related to treatment
No death observed
Renal function
- Of the 24 patients with CKD stage 3b or 4 and with available results, eGFR remained unchanged from screening to end of treatment and post-treatment week 4: 27.1 ± 9.2 vs 26.4 ± 9.8 vs 27.4 ± 11.6 mL/min/1.73m²
- CKD stage remained unchanged in 22/24 patients with end of treatment results and declined in 2/24 from screening to end of treatment
Summary
- GLE/PIB is highly efficacious in patients with chronic kidney disease stage 3b to 5 with the label recommended treatment durations based on genotype, cirrhosis status and prior treatment experience
Treatment was well-tolerated - Overall, renal function remained unchanged after treatment in pre-dialysis patients assessed